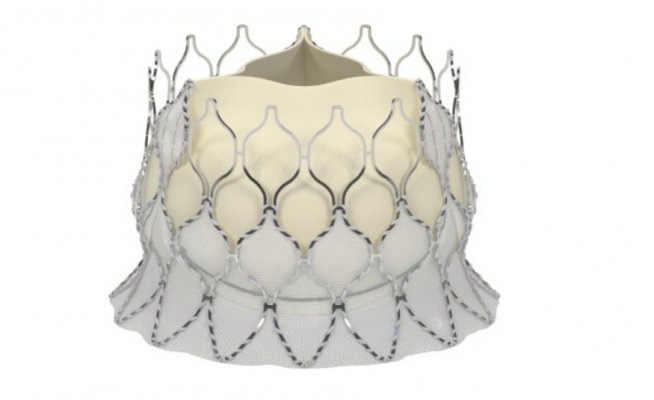
May 24, 2017 — Late-breaking data presented at EuroPCR 2017 demonstrating excellent clinical outcomes for transcatheter aortic valve replacement (TAVR) patients treated with the new self-expanding Edwards Lifesciences’ Centera valve. These new 30-day results are the first data presented on the self-expanding Edwards Centera valve.
For severe, symptomatic aortic stenosis patients at high risk of open-heart surgery, the Edwards Centera valve demonstrated a very high survival rate of 99 percent, a low 2.5 percent disabling stroke rate and a 4.9 percent permanent pacemaker rate – the lowest rate ever reported in a multi-center trial for a self-expanding valve. Additionally, there was a low 0.6 percent rate of moderate paravalvular leak among patients, and no severe paravalvular leak. All 203 patients in the study were treated via the transfemoral access route with the majority (174 patients) under conscious sedation.
The Centera valve is repositionable and retrievable and can be delivered through a low-profile 14-French delivery system that features a motorized handle that results in stable valve deployment. The valve is uniquely packaged – fully pre-attached which facilitates simple and rapid device preparation.
"The Edwards Centera valve demonstrates extremely favorable early clinical safety and performance outcomes in the high surgical risk TAVR population," said Didier Tchétché, M.D., of Clinique Pasteur in Toulouse, France, who presented the data. "In addition to excellent patient outcomes, the valve also offers several unique features and an innovative tissue design, all of which simplify the procedure for clinicians."
CENTERA-EU Trial patients were enrolled in 23 centers in Europe, Australia and New Zealand and will be followed for five years. The Edwards Centera valve is an investigational device not yet available commercially in any country. Edwards anticipates it will receive CE mark approval for the Centera valve during the fourth quarter of 2017.
Tchétché is a consultant to Edwards Lifesciences.
For more information: www.Edwards.com


 November 14, 2025
November 14, 2025 









