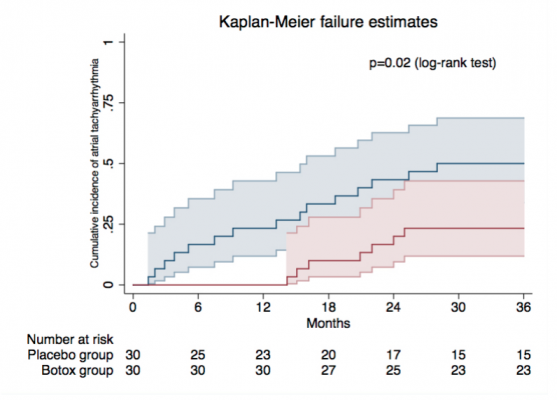
Figure 1: At the end of 36 months, the incidence of any atrial tachyarrhythmia was 23.3 percent in the botox group, as compared to 50 percent in the placebo group
May 18, 2018 — Three year results of a study found injection of botulinum toxin into epicardial fat pads in patients undergoing coronary artery bypass grafting (CABG) has resulted in substantial atrial fibrillation (AF) suppression. The results were presented at Heart Rhythm 2018, the Heart Rhythm Society’s 39th Annual Scientific Sessions.
There was sustained substantial reduction of atrial tachyarrhythmia incidence and AF burden in the early post-operative period, at one-year and three-year follow-up, data show.[1] This was accompanied by reduction in hospitalizations and major clinical adverse events. A large-scale multicenter randomized trial is needed to focus on hard clinical outcomes to more comprehensively test the value of botulinum toxin injections during cardiac surgery.
The study included 60 patients with history of paroxysmal AF and indications for CABG were randomized to either botulinum toxin (50U/1 ml at each fat pad; botox group; n=30) or placebo (0.9 percent normal saline, 1 ml at each fat pad; placebo group n=30) injections into four posterior epicardial fat pads. All patients received an ICM with regular follow-up. The primary endpoint of the extended follow up was incidence of any atrial tachyarrhythmia including AF and atrial tachycardia after 30 days of procedure until 36 months on no antiarrhythmic drugs. The secondary endpoints included clinical events and AF burden.
At the end of 36 months, the incidence of any atrial tachyarrhythmia was 23.3 percent in the botox group, as compared to 50 percent in the placebo group (hazard ratio 0.36, 95 percent confidence interval 0.14-0.88, p= 0.026) See figure 1. The three-year AF burden was significantly lower in the botox group compared to the placebo group: 1.4 vs 6.9 percent (p < 0.001). In botox group, two (7 percent) patients were hospitalized during follow-up compared to 10 (33 percent) in placebo group (p=0.02); and there were no major clinical adverse events in botox group versus four patients (13 percent) in placebo group who developed stroke or died (p=0.1).
Find links to all the Heart Rhythm 2018 Late-breaking Studies
Reference:


 December 19, 2025
December 19, 2025 









