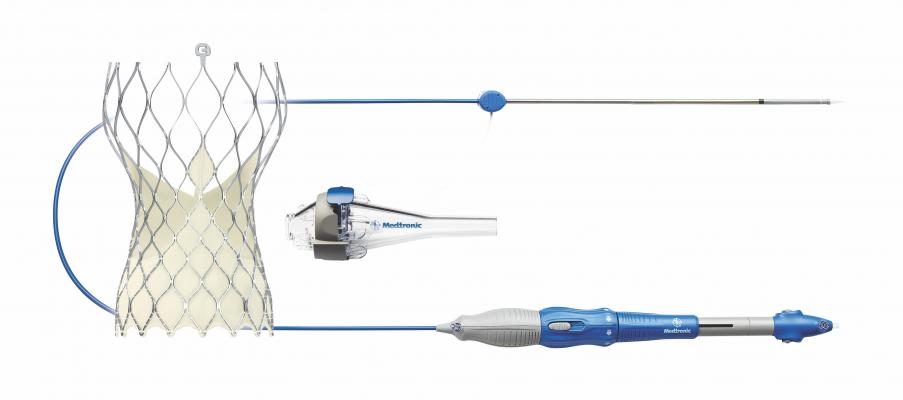
September 19, 2018 – The U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) to initiate a new single-arm study of Medtronic’s CoreValve Evolut transcatheter aortic valve replacement (TAVR) system in patients with bicuspid aortic valves. All patients in the study will be at low risk of surgical mortality. Medtronic separately received FDA approval for revised commercial labeling for the CoreValve Evolut TAVR system that removed a precaution for the treatment of bicuspid severe aortic stenosis patients deemed at intermediate or greater risk for surgical aortic valve replacement.
“Real-world data suggests that TAVR with the self-expanding Evolut can be a suitable treatment option for many patients with bicuspid aortic valve disease,” said Jeffrey J. Popma, M.D., director of interventional cardiology at the Beth Israel Deaconess Medical Center in Boston. “In fact, data from the TVT [Transcatheter Valve Therapy] Registry has shown near-parity in certain outcomes between bicuspid and tricuspid patients using the Evolut self-expanding platform.”
Estimated to affect 1 in 5 patients undergoing surgical aortic valve replacement (SAVR), bicuspid valve patients are born with two aortic valve leaflets instead of the more common three leaflets (tricuspid). While the revised labeling approval pertains to patients deemed at intermediate risk or greater for SAVR, Medtronic is studying bicuspid patients within a separate single-arm study of the Low Risk TAVR Trial. In the U.S., use for treatment with bicuspid aortic valves in patients who are at low risk of surgical mortality is investigational use only.
Medtronic said the label revision enables the company to provide proactive training and education on procedural TAVR sizing and placement in this particular patient population.
For more information: www.medtronic.com


 January 05, 2026
January 05, 2026 









