November 6, 2018 — 4Tech Inc. initiated its U.S. Early Feasibility Study of the TriCinich Coil System following U.S. Food and Drug Administration (FDA) approval, with the first two implantations at Piedmont Heart Hospital in Atlanta. The implantations were conducted by Christopher Meduri, M.D., Vivek Rajagopal, M.D., and Mani Vannan, M.D. The study will evaluate the TriCinch Coil System in 15 patients across seven centers in the U.S.
“We are pleased to be the first center in the U.S. to implant the TriCinch Coil System,” said Meduri. “Both procedures went smoothly, and the device was easy to implant. Having a technology like the TriCinch Coil System in our structural heart toolkit allows us to treat a wide range of patients suffering from tricuspid regurgitation, who are at high risk for open heart surgery, in a safe and simple manner.”
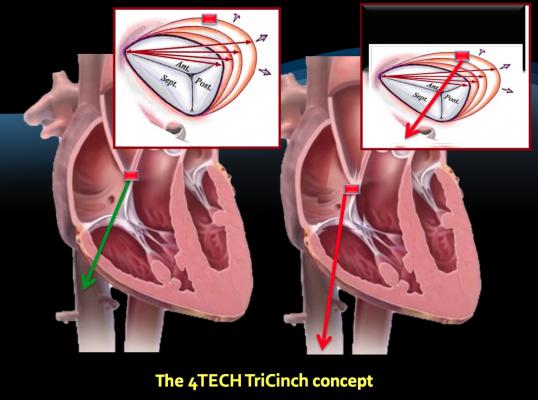
The 4Tech TriCinch Coil System is a percutaneous direct annuloplasty device designed to reduce tricuspid regurgitation by means of tricuspid valve (TV) remodeling via a nitinol coil anchor that is tensioned by a nitinol stent in the inferior vena cava (IVC).
The device is being evaluated worldwide with a CE-Trial that has enrollment in both Australia and Europe.
Tricuspid regurgitation is a common condition that is age-related and difficult-to-manage, in which blood “backflows” into the right atrium. Today’s standard of care for TR is medical management. Surgical intervention is high-risk, with an in-hospital mortality for isolated TR as high as 37 percent1. Patients with TR and related complications result in substantial healthcare spending due to frequent rehospitalizations. Furthermore, TR leads to chronic renal failure and the need for end-stage dialysis. The combination of these negative outcomes results in a significant unmet need for a minimally invasive solution for TR.
For more information: www.4techtricuspid.com
Reference
1. Fender E.A., et al. Heart 2017;0:1–9. doi:10.1136/heartjnl-2017-311586


 November 14, 2025
November 14, 2025 









