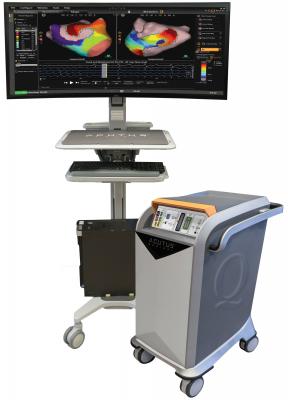
April 26, 2019 — Acutus Medical announced U.S. Food and Drug Administration (FDA) clearance of its second-generation AcQMap platform, along with CE Mark approval for its AcQMap contact mapping software.
AcQMap offers expanded functionality that provides physicians with more options to inform individualized patient therapy. All AcQMap systems retain the ability to quickly reconstruct atrial anatomy using ultrasound and map electrical patterns using non-contact, charge density technology, creating ultra-high-resolution 3-D images in real time.
"The contact mapping speed with the new Acutus system was extremely fast, which made it very easy to use," said Christian Meyer, M.D., University Medical Center Hamburg-Eppendorf, Hamburg, Germany. "Clinically, having this capability available on one system that also does non-contact mapping allows me to do exactly what makes sense case by case. For routine cases, my treatment strategy can be confirmed using conventional mapping catheters. In more complex cases, such as atrial fibrillation, I can gather more comprehensive data about each patient's anatomy and arrhythmia in real time with the non-contact charge density catheter, making AcQMap the complete atrial mapping solution."
Cardiac ablation treatment can improve quality of life for patients with atrial arrhythmias such as atrial fibrillation (AF). AF affects more than 33 million people globally, and increases the risk of stroke and heart failure.1,2 AcQMap enables physicians to uncover the electrical activation pattern of the heart with real-time visualization during electrophysiology procedures. With non-contact mapping, physicians see full-chamber, continuous beat-by-beat arrhythmia conduction and can locate arrhythmias outside the pulmonary veins to inform their treatment strategy, enabling an iterative map-ablate-remap approach.
Acutus Medical's contact mapping software is compatible with both first- and second-generation AcQMap platforms and will be installed on all European systems in Q3 2019. The second-generation AcQMap system is built to support future software innovations and will be available in the U.S. in Q3 2019.
For more information: www.acutusmedical.com
References


 February 06, 2026
February 06, 2026 









