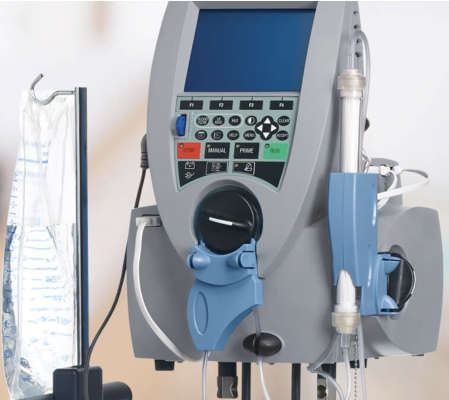
September 7, 2022 — Nuwellis, Inc., a commercial-stage company focused on transforming the lives of people with fluid overload, announced the publication of key clinical data supporting the use of ultrafiltration with the Aquadex FlexFlow System in high-risk postoperative coronary artery bypass grafting (CABG) patients in the Journal of Cardiac Surgery.
The study, “The Use of Simple Ultrafiltration Technology as a Fluid Management Strategy for High-Risk Coronary Artery Bypass Grafting Surgery,” is a real-world retrospective review of postoperative isolated CABG patients who were treated in the Division of Cardiothoracic Surgery at Baylor Scott & White Health in Temple, Texas between January 1, 2020 and July 31, 2021. A total of 254 CABG procedures were performed during this period, and ultrafiltration was used in 17 (6.7%) patients. Despite an elevated mean Society of Thoracic Surgeons mortality score of 5.7% (range 0.6-50.0), the 30-day survival rate of patients treated with ultrafiltration was 100%.
“Ultrafiltration technology has been used for decades to successfully treat advanced heart failure patients and data hav been published to demonstrate its outcomes, but its application in cardiac surgery to treat fluid overload is innovative,” said Daniel Beckles, M.D., Surgical Director of Heart Transplantation and Mechanical Circulatory Support at Baylor Scott & White Health and the study’s lead author. “These favorable results are consistent with the clinical findings in other large academic centers using ultrafiltration technology in cardiac surgery, and we are eager to gather additional multi-institutional data about the use of Aquadex for this challenging patient population.”
According to the Society of Thoracic Surgery Adult Cardiac Surgery Database, isolated CABG for coronary artery disease (CAD) is the most commonly performed cardiac surgery procedure, with over 162,000 per year.1 The increased age of patients, in addition to risk factors for CAD (hypertension, diabetes, obesity and chronic kidney disease), increases the perioperative risk for acute kidney injury and subsequent fluid overload. Excess fluid overload in the perioperative phase of care has been associated with increased morbidity and mortality for CABG patients.2
“We are pleased with the results of this first-of-its-kind study highlighting the benefits of Aquadex therapy for high-risk CABG patients,” said Nestor Jaramillo, Jr., President and CEO of Nuwellis. “The Aquadex System offers a significant opportunity to the medical community to improve clinical outcomes. In 2019, we made the decision to expand our strategic focus to include the use of Aquadex in critical care (cardiac surgery) and pediatric settings. Today, critical care represents approximately 40% of our total revenue, confirming the execution of the strategy and the clinical value of the Aquadex System in this patient segment.”
In addition to cardiovascular surgery, Nuwellis continues to make significant progress in other key areas of patient need, including heart failure and pediatrics. The company recently announced the first patient enrolled in its REVERSE-HF clinical study, which is evaluating the clinical outcomes and economic value of its Aquadex® ultrafiltration therapy in comparison to intravenous (IV) diuretics for the treatment of fluid overload in patients with worsening heart failure. Nuwellis is also developing a new, fully integrated pediatric continuous renal replacement therapy (CRRT) device designed to provide care for small babies and children. This device is being funded in part by a $1.7 million grant from the National Institutes of Health (NIH). Nuwellis has partnered with Minneapolis-based research and development firm Koronis Biomedical Technologies Corporation (KBT), the grant recipient, to design and develop a custom pediatric product that will enable clinicians to better care for babies with limited kidney function.
For more information: https://ir.nuwellis.com/
References:
1 Bowdish ME, D’Agostino RS, Thourani VH, Schwann TA, Krohn C, Desai N, Shahian DM, Fernandez FG, Badhwar V. STS Adult Cardiac Surgery Database: 2021 Update on Outcomes, Quality, and Research. Ann Thorac Surg 2021;111:1770-80.
2 Pal S. Primary Causes of End-Stage Renal Disease. US Pharm. 2016;41(8):6.


 February 03, 2026
February 03, 2026 









