March 22, 2023 — Windtree Therapeutics, Inc., a biotechnology company focused on advancing multiple late-stage interventions for cardiovascular disorders, announced that the United States Patent and Trademark Office (USPTO) has issued U.S. Patent No. 11,583,540, providing expanded patent coverage for istaroxime administration.
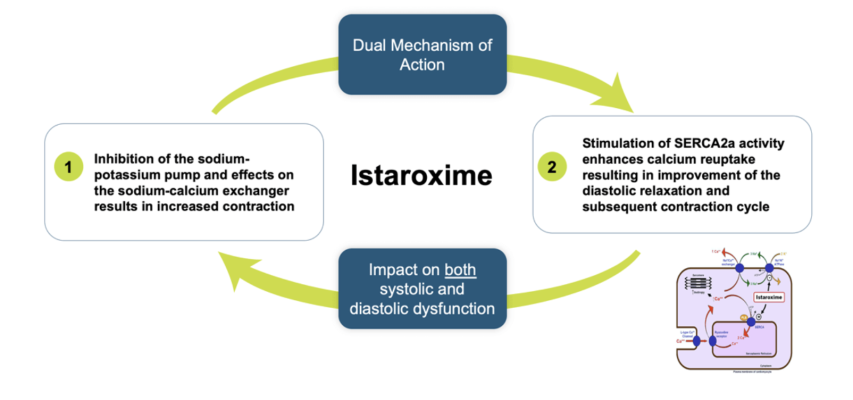
Istaroxime is an investigational drug candidate being studied in early cardiogenic shock and acute heart failure. It has a novel dual mechanism of action that increases both systolic contraction and diastolic relaxation, uniquely addressing heart failure associated SERCA2a dysfunction. SERCA2a activation facilitates sequestration of calcium during diastole, favoring relaxation and making more calcium available for the next contraction, further improving cardiac ventricular function. Phase 2a and Phase 2b studies in acute heart failure have demonstrated significant improvements in cardiac function as well as increasing blood pressure and preserving or increasing renal function.
The new U.S. patent, titled: “Istaroxime-Containing Intravenous Formulation for the Treatment of Acute Heart Failure (AHF)”, is a continuing patent following the expedited U.S. Track One filing by Windtree, which resulted in U.S. Patent No. 11,197,869 that issued December 14, 2021. The claims of the newly issued patent cover longer durations of istaroxime infusion for improved outcomes in treatment of acute heart failure. In particular, the claims are directed to an improvement in diastolic heart function following administration of istaroxime by intravenous infusion for 6 hours or more, which Windtree attributes to the SERCA2a mechanism of action of istaroxime and its metabolites.
“Windtree continues to strengthen the patent estate for istaroxime with this new issuance from the USPTO,” said Craig Fraser, CEO and President of Windtree Therapeutics. “We believe istaroxime is differentiated from other drugs that are used to treat acute decompensated heart failure and this patent provides additional rationale for how it can provide unique benefits to potential patients. We plan to continue this work to further expand istaroxime’s patent estate in the clinical development work ahead.”
For more information: https://windtreetx.com/
Related Content:
Windtree Completes Enrollment of Phase 2 Study of Istaroxime in Early Cardiogenic Shock


 January 05, 2026
January 05, 2026 









