Getty Images
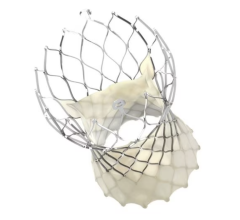
November 20, 2023 — A groundbreaking advancement in the treatment of patients with uncontrolled hypertension (HTN) has been achieved with the recent U.S. Food & Drug Administration (FDA) approvals of two renal denervation (RDN) systems. These innovative systems, developed by leading medical technology companies Medtronic, Inc (Symplicity Spyral Renal Denervation System) and ReCor Inc. (Paradise Ultrasound Renal Denervation System), have the potential to transform the lives of individuals suffering with uncontrolled HTN, who may be inadequately responsive to, or are intolerant to, anti-hypertensive medications.
The FDA's approval of these RDN systems marks a significant milestone in the field of cardiovascular medicine. This invasive procedure offers a promising therapy to this targeted population. By targeting the nerves surrounding the kidneys, the systems disrupt the signals that contribute to high blood pressure, providing patients with a potentially life-changing solution.
"The approval of the renal denervation systems by the FDA is a game-changer for both interventional cardiology and the treatment of hypertension," said Dr. George Dangas, president of SCAI. "This innovative technology has the potential to revolutionize how we approach the management of high blood pressure which has grown tremendously globally, offering patients a safe and effective treatment option."
Hypertension, commonly known as high blood pressure, affects approximately one in three adults worldwide and is a leading cause of heart disease, stroke, and kidney failure. Despite the availability of various treatment options, many patients struggle to achieve adequate blood pressure control.
The FDA's approval of the RDN systems comes after the Circulatory System Devices Panel hearing in August and was based on clinical trials demonstrating its safety and efficacy. The ReCor, Inc.’s Paradise Ultrasound RDN System was supported by data provided by the RADIANCE program. RADIANCE II and RADIANCE-HTN SOLO studied patients with mild to moderate hypertension in an "off-meds" setting, and RADIANCE-HTN TRIO enrolled patients with resistant hypertension on standardized triple anti-hypertensive therapy. Medtronic, Inc.’s Symplicity spiral was supported by data from the SPYRAL HTN-ON MED which evaluated the effect of the Symplicity blood pressure procedure in the presence of anti-hypertensive drugs and the HTN-OFF MED trial which examined patients with uncontrolled hypertension in the absence of anti-hypertensive medications.
Earlier this summer, SCAI released a position statement on RDN for HTN regarding patient selection, best practices for optimal techniques, competence, training and organizational recommendations. In the statement, SCAI states that appropriate patient selection, pre-procedure evaluation, careful procedural planning and technique, implementation of strict operator training standards and facility requirements are paramount to programmatic success. SCAI also led a webinar on the topic and plans to host a workshop on building a successful RDN program at SCAI 2024 Scientific Sessions.
For more information: www.scai.org


 May 05, 2025
May 05, 2025