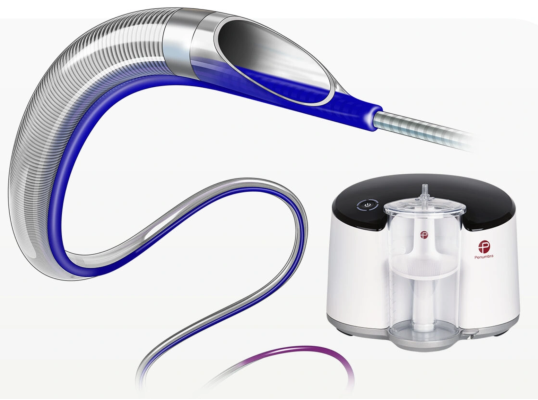
January 16, 2024 — Penumbra, Inc., a thrombectomy company, has recently secured CE Mark (Conformité Européenne) for its Indigo System CAT RX designed to address the limitations of traditional management strategies in acute coronary syndrome (ACS). Traditional modalities utilizing syringe aspiration suffer from diminished vacuum once fluid enters the system. Now commercially available in Europe, CAT RX is designed to navigate tortuous coronary anatomy while maintaining sustained mechanical aspiration with the Penumbra ENGINE.
“Expanding access of CAT RX in Europe will have a significant impact on the high-risk coronary patient population,” said Joan Kristensen, vice president and head of the Europe, Middle East and Africa region for Penumbra, Inc. “In what is often a race against time, CAT RX designed for speed, safety and simplicity will allow physicians to restore blood flow in acute MI patients.”
As shown in the CHEETAH study, CAT RX demonstrated high rates of blood clot removal, blood flow restoration and myocardial perfusion in conjunction with percutaneous coronary intervention (PCI) in patients with high thrombus burden.
“The CHEETAH findings suggest that continuous aspiration with CAT RX should be a key consideration when high thrombus burden plaque is encountered given that removing the thrombus improves perfusion. CAT RX has demonstrated a high degree of safety while maximizing effectiveness in removing thrombus. We believe this can lead to better outcomes for the patient,” said James F. Benenati, M.D., FSIR, chief medical officer at Penumbra.
For more information: https://www.penumbrainc.com/products/coronary-thrombectomy-cat-rx/


 November 14, 2025
November 14, 2025 









