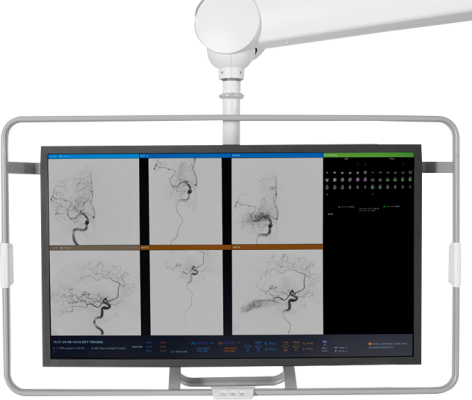
May 7, 2024 — RapidAI, the global leader in developing clinically deep Artificial Intelligence (AI) and technology workflow solutions to combat life-threatening neurovascular, cardiac, and vascular diseases, today announced that the Food and Drug Administration (FDA) has granted 510(k) clearance for its AngioFlow by RapidAI solution. The module delivers perfusion imaging analysis directly within the interventional suite to promote greater clinical confidence, workflow efficiency, and potentially improve patient outcomes.
“We are pleased to expand our stroke AI imaging portfolio, the broadest in the industry, to include this new module. With AngioFlow by RapidAI, we can now support stroke AI imaging along the entire patient pathway, from the initial non-contrast CT scan all the way to the interventional suite,” said Karim Karti, CEO of RapidAI. “Already registered and used in Japan and Europe, we believe this technology will make a significant difference in the lives of U.S. stroke patients.”
In acute stroke care where time is brain, timely intervention can be not only life-saving but can significantly reduce the risk of long-term disability. Used by care teams working in the interventional suite, AngioFlow by RapidAI draws on validated deep clinical AI to deliver clear, easy-to-interpret qualitative perfusion maps within minutes to help specialists assess ischemic change in brain regions with reduced cerebral blood flow.
AngioFlow by RapidAI enhances operational efficiency and financial value by reducing redundant imaging and potentially saving time in patient care. Imaging at a referring facility allows physicians to evaluate the need for further scans in the interventional suite, accelerating clinical decisions and reducing unnecessary imaging. This is especially important in rural facilities and large hospital networks, where transfers may take hours to get from one facility to the next.
“AngioFlow by RapidAI will allow physicians to assess the need for additional imaging immediately in the interventional suite. By avoiding unnecessary scans, stroke patients can receive the timely care that can be the difference between being able to walk out of a hospital to their homes versus being discharged to a skilled nursing facility,” said Abhishek Singh, MD, DABPN, DUCNS at the Creighton University School of Medicine in Omaha, Nebraska.
For more information: https://www.rapidai.com/


 November 14, 2025
November 14, 2025 









