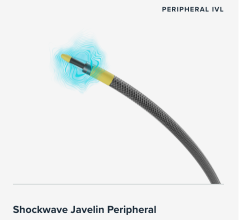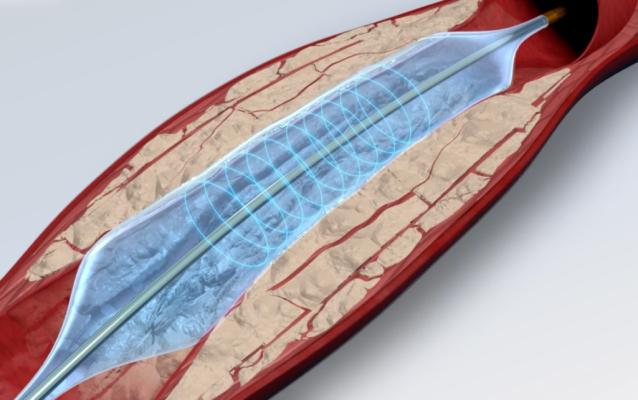
Sept. 9, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on safely and effectively treating severely calcified arterial disease, will present 30-day results of its POWER PAD II U.S. pivotal trial for Pulsatile Intravascular Lithotripsy (PIVL) therapy as a Late Breaking Clinical Trial (LBCT) at the Vascular InterVentional Advances (VIVA) meeting in November in Las Vegas.
The POWER PAD II clinical study was designed to evaluate the safety and efficacy of AVS’s Pulse IVL System for treating patients with moderate to severely calcified peripheral arterial disease in the United States. Chris Metzger, M.D., Interventional Cardiologist at Ballad Health in Kingsport, Tenn., is the National Principal Investigator of the POWER PAD II Trial.
“AVS is the only company to conduct an Above-The-Knee (ATK) Intravascular Lithotripsy pivotal IDE study in the U.S., and we are excited to present our initial findings at the Late Breaking Clinical Trial session at VIVA,” said Elizabeth Galle, Vice President of Clinical Affairs at AVS. “This marks our most significant clinical milestone to date as we approach FDA clearance and market availability for the Pulse IVL™ System. We are confident in our innovative technology, which is designed for easy delivery across complex calcified lesions.”
“It has been a distinct pleasure overseeing this trial, in collaboration with our study investigators and more importantly, the patients who have benefited from this innovative approach to Intravascular Lithotripsy. I received feedback from several of our investigators that they appreciated the procedural efficiency due to its improved deliverability and fast therapeutic delivery,” said Dr. Metzger.
“By introducing a new, innovative treatment for calcified arterial disease, we can make a dramatic impact on patient lives and improve outcomes,” said Mark Toland, Chairman of AVS. “The results of this pivotal trial will pave the way for FDA 510k clearance and commercial availability of a new treatment option in an evolving and exciting area of medicine: IVL therapy.”
To learn more about AVS and the PULSE IVL System, please visit www.avspulse.com.


 November 09, 2025
November 09, 2025