The newly appointed chief of cardiovascular medicine and co-director of the Nicklaus Children’s Heart Institute, Dr. Shyam Sathanandam, is the pioneer of the minimally invasive technique now adopted by Nicklaus Children’s.
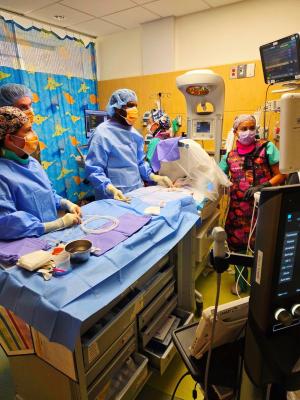
Sept. 22, 2025 — Nicklaus Children's Heart Institute in Miami, Florida, is now offering bedside transcatheter patent ductus arteriosus (PDA) closures for medically fragile newborns. This new offering brings advanced therapy directly to the neonatal intensive care unit, allowing procedures to be performed within the baby's own isolette or crib. This eliminates the need to transport the infant to a catheterization laboratory or even outside of their NICU room, removing any risks associated with moving fragile babies.
A PDA is a small blood vessel that normally closes after birth. In some premature infants, the vessel remains open, allowing extra blood to flow to the lungs. This can lead to breathing difficulties, feeding challenges, growth delays, and other complications. In cases where a PDA does not respond to medication or close on its own, closure may be needed to stabilize the infant's condition. The minimally invasive procedure involves placing a small catheter through a vein to deliver a tiny device that seals the PDA from within.
The newly appointed chief of cardiovascular medicine and co-director of the Nicklaus Children's Heart Institute, Dr. Shyam Sathanandam, is the pioneer of the minimally invasive technique now adopted by Nicklaus Children's. Under his leadership, the Heart Institute is on track to become the first hospital in the nation to offer a traveling PDA closure program for children admitted at other hospitals throughout South Florida.
"Offering bedside transcatheter PDA closures means South Florida families no longer need to see their fragile newborns transferred out for this procedure," said Dr. Shyam Sathanandam. "By bringing the catheterization team and technology directly to the NICU, we reduce transport-related risks and deliver state-of-the-art cardiac care right where these babies are safest—next to the clinicians and equipment they depend on."
Benefits of bedside PDA closures include eliminating the need for transport of fragile infants out of the NICU, a minimally invasive approach with no chest incision, and support for faster recovery from a collaborative team of clinical professionals including, neonatologists, cardiologists, anesthesiologists and nursing experts at the bedside.
Not all PDAs require closure. Each baby is carefully evaluated by a multidisciplinary team to determine whether observation, medication, or closure is the best option, and families are included in every step of the process, with treatment tailored to each infant's unique needs.
The bedside PDA closure program at Nicklaus Children's Heart Institute builds on the comprehensive spectrum of pediatric and congenital cardiac care, offering families in South Florida access to advanced therapies close to home.
The Nicklaus Children's Heart Institute is internationally recognized for its expertise in pediatric cardiology and cardiac surgery, caring for thousands of children each year with congenital and acquired heart conditions.
The state-of-the-art Pediatric and Congenital Catheterization Program at Nicklaus Children's is widely recognized as one of the world's leaders, utilizing the latest innovations in imaging and device technologies to perform both simple and complex interventions that can reduce – or even eliminate – the need for open-heart surgery.
For more information about Nicklaus Children's Heart Institute, visit www.nicklauschildrens.org/heart.


 November 14, 2025
November 14, 2025 









