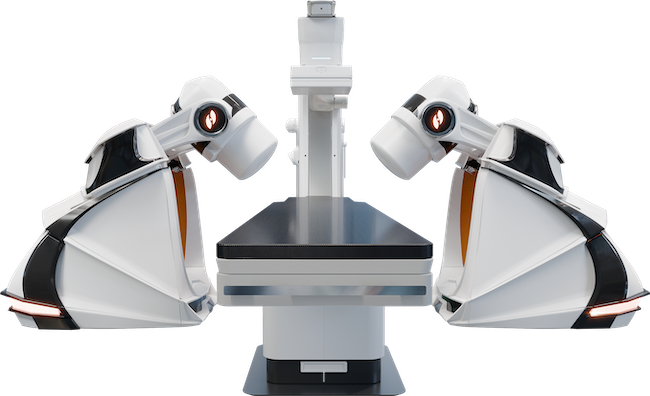
The GenesisX Robotic Magnetic Navigation system is semi-mobile and can be set up in a catheterization lab over the weekend. Photo: Stereotaxis
Robotic Magnetic Navigation (RMN) emerged two decades ago as an alternative approach to performing complex ablation procedures inside cardiac catheterization and electrophysiology (EP) labs. Several meta-analyses link RMN to lower complication rates and less fluoroscopy time compared to manual navigation approaches.1,2 However, implementing RMN platforms has traditionally required significant architectural planning and other hurdles, limiting their adoption and making them out of reach for many community hospitals.
To address this challenge, Stereotaxis, a pioneer in RMN technology for cath and EP labs, is moving toward smaller, more capital-efficient robotic solutions with its GenesisX RMN platform. FDA-cleared in November 2025,3 GenesisX is roughly half the size of the company’s Niobe system, which has been used successfully for the past two decades.
“[With GenesisX] you don’t need to do any magnetic shielding in the walls of the room,” says David Fischel, CEO of Stereotaxis. “You don’t need to reinforce the floor. You can use normal power outlets. And the system overall takes less space, allowing hospitals to place the robot in existing cath labs without construction challenges.”

Fulfilling a Growing Need
The move toward smaller robotic systems comes as the number of EP procedures continues to climb. Fischel estimates that volumes are growing between 10% to 20% nationwide, sparked in part by the rising incidence of cardiac arrhythmias like atrial fibrillation (AFib), which affects approximately 10.5 million U.S. adults.4
RMN is designed to help physicians treat complex arrhythmias with more precision. Instead of using a rigid catheter, RMN systems navigate a flexible catheter with a magnetic tip. Interventionalists control the procedure from a computer cockpit, manipulating robotically actuated magnets located on either side of the patient to help guide the catheter.
“This mechanism of action gives physicians millimeter control over a flexible catheter that’s gentle and atraumatic, allowing them to reach areas of the heart that aren’t possible to access with manual catheters,” Fischel says.
Stereotaxis’ product portfolio also includes two other next-generation catheters. One, MAGiC Sweep, is a diagnostic model that uses 20 electrodes to create a highly detailed electrical map of the heart’s chambers, helping physicians pinpoint the source of an arrhythmia. The other, MAGiC, is an irrigated therapeutic ablation catheter that uses radiofrequency to treat arrhythmias. MAGiC Sweep received FDA clearance in July 20255; MAGiC received FDA clearance on Jan. 6, 2026.6
Changing EP Lab Workflows
Fischel says, robotics can help increase the efficiency of cath and EP lab workflows by giving individual operators the opportunity to take more control over procedures without needing as much clinical support.
“We have this beautiful image — and there are a dozen or so physicians working like this now — of a physician who’s seated at the computer doing a procedure,” he says. “He’s wearing socks. He has three pedals at his legs, one hand on a [computer] mouse, one hand on the keyboard, and he’s doing the entire procedure alone, with just one nurse who’s walking between the patient and the control room.”
RMN also simplifies the mechanical aspects of an ablation procedure, according to Fischel. “When you make the mechanical part of a procedure easier, physicians can spend more of their energy on the cognitive side, which I think is part of the beautiful evolution of medicine that plays out as you expand robotics,” he says.
Moving Toward Digital Surgery
Adding digital capabilities to RMN, Fischel says, will expand access even further. To that end, Stereotaxis has developed another product, Synchrony & Synx, a digital cockpit that consolidates all cath and EP lab systems onto a single display, enabling remote collaboration between cath labs and physicians across regions. Synchrony is currently approved for use in Europe; U.S. approval is pending.7
“We’ve watched robotics have major impacts on laparoscopic and orthopedic surgeries,” Fischel says. “The cath lab deserves to have that technological progress as well. That’s our mission for the coming years, to make robotics a positive, impactful technology across interventional medicine.”
References
-
Virk SA, Ariyaratnam J, Bennett RG, et al. Updated systematic review and meta-analysis of remote magnetic navigation versus manual navigation for atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2019;12(10):e007517. doi:10.1161/CIRCEP.119.007517
-
Ijaz R, Singh A, et al. Comparison between efficacy and safety of remote magnetic navigation and manual catheter navigation for atrial fibrillation ablation: an updated meta-analysis and systematic review. J Innov Card Rhythm Manage. 2025;16(6):6307–6328. doi:10.19102/icrm.2025.160601
-
U.S. Food and Drug Administration. 510(k) Premarket Notification: Stereotaxis GenesisX RMN with Navigant™ Workstation (NWS). K251792. Published Nov. 6, 2025. Accessed Jan. 3. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K251792
-
Cheung JW, Cheng A, Cheng EP, et al. Prevalence and incidence of atrial fibrillation in the United States, 2012-2022. J Am Coll Cardiol. 2024;84(13):1176–1186. doi:10.1016/j.jacc.2024.07.014
-
Stereotaxis receives U.S. FDA clearance for MAGiC Sweep™ catheter [press release]. St. Louis, MO: GlobeNewswire; July 28, 2025. Accessed Jan. 3. https://www.globenewswire.com/news-release/2025/07/28/3122378/21634/en/Stereotaxis-Receives-U-S-FDA-Clearance-for-MAGiC-Sweep-Catheter.html
-
Stereotaxis receives FDA approval for MAGiC ablation catheter. Stereotaxis Inc [press release]. Published Jan. 6. Accessed Jan. 7. https://ir.stereotaxis.com/news-releases/news-release-details/stereotaxis-receives-fda-approval-magic-ablation-catheter
-
>Stereotaxis announces EU launch and 510(k) submission for Synchrony™ [press release]. St. Louis, MO: Stereotaxis, Inc.; May 13, 2024. Accessed Jan. 3. https://ir.stereotaxis.com/news-releases/news-release-details/stereotaxis-announces-eu-launch-and-510k-submission-synchrony


 November 14, 2025
November 14, 2025 









