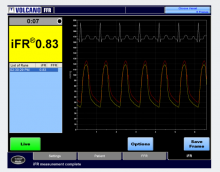
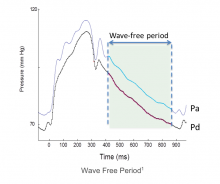
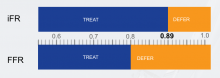
Earlier this year, the Barnes-Jewish Hospital at Washington University School of Medicine in St. Louis adopted the instant wave-Free Ratio (iFR) technology. The iFR modality is a pressure-derived hyperemia-free index for assessment of coronary stenosis relevance.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now