By Dave Fornell, DAIC editor
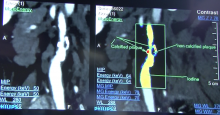
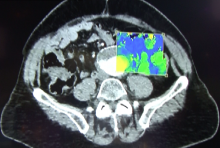
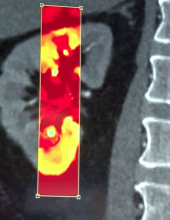
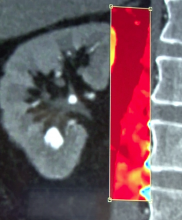
While the Radiological Society of North America (RSNA) focuses on new technology for radiology, it is where vendors formally introduce all their imaging systems, including those for cardiology. The annual RSNA meeting is also a barometer for new trends in imaging and imaging related information technology.