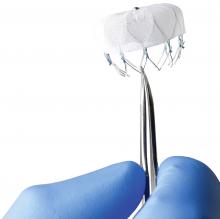
At RSNA 2013, GE Healthcare introduced the Discovery Interventional Guidance System (IGS) 740, a 510(k)-pending mobile X-ray angiography system with a 41 x 41 cm detector. The system is aimed at interventional radiology market.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now