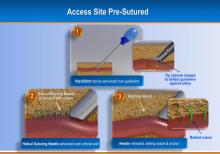
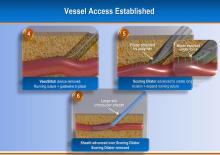
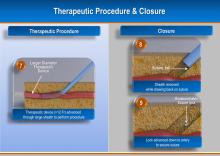
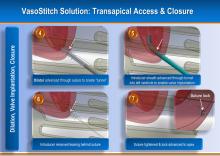
August 17, 2012 — Jarvik Heart Inc., a privately held company that develops and manufactures cardiac assist devices, announced U.S. Food and Drug Administration (FDA) approval of its pivotal trial for evaluation of the Jarvik 2000 heart for destination therapy (DT), named RELIVE (Randomized Evaluation of Long-term Intraventricular VAD [ventricular assist device] Effectiveness).
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now