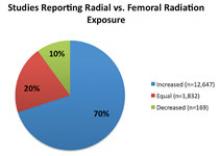
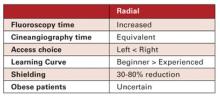
Vascular closure devices that use an active method to immediately seal the femoral access site can enable faster patient ambulation, reduce nursing time and speed discharge. However, one of the biggest issues interventionalists have with active vascular closure devices is the use of a permanent piece of hardware to stitch or clip the arteriotomy closed. Three companies now offer fully bioresorbable, active vascular closure devices, including a recent release earlier this year.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now