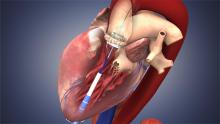
June 14, 2012 — A U.S. Food and Drug Administration (FDA) advisory panel voted in favor of recommending expanding the indication for the Edwards Lifesciences Sapien transcatheter heart valve for the treatment of high-risk patients. Currently, the Sapien is only indicated for patients who are too sick to undergo valve replacement surgery for severe, symptomatic aortic stenosis. The panel voted 11-0, with one abstention, that the benefits of the heart valve outweighed the risks for these patients.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now