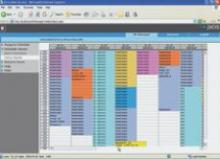
When diagnosing trauma patients, speed and accuracy is essential. As a result, there is a movement away from traditional film to digital X-ray to make images immediately available to doctors anywhere in the hospital, or even on computers outside the hospital
If you enjoy this content, please share it with a colleague
- Read more about Digital Versus Computed Radiology
- Log in or register to post comments