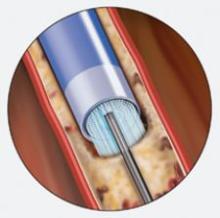
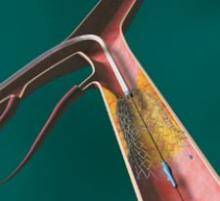
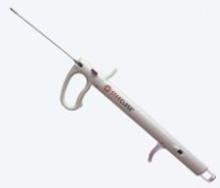
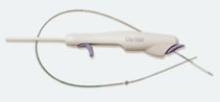
Abiomed Inc. recently announced the start of the IMPELLA 2.5 pilot study in the U.S. with the enrollment of the first patient at William Beaumont Hospital in Detroit, MI. The IMPELLA 2.5 is the world’s smallest ventricular assist device (VAD) and is currently available in Europe under the CE Mark.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now