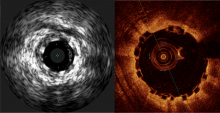
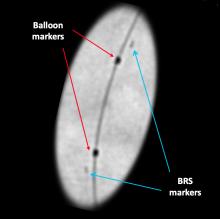
Some have labeled bioresorbable scaffolds (BRS), also known as bioresorbable stents, as the fourth evolution of interventional cardiology. These novel devices follow the successive evolutions of angioplasty, bare metal stents (BMS) and drug-eluting stents (DES), each of which led to a marked improvement in clinical outcomes for patients suffering from coronary artery disease (CAD). The most exciting aspect of BRS is preliminary data shows the vessel is healing, and histologically looks nearly normal approximately three years after scaffold implantation.
If you enjoy this content, please share it with a colleague
- Read more about Role of Imaging in Bioresorbable Stent Procedures
- Log in or register to post comments