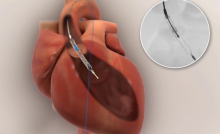
Direct Flow Medical Inc. received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) in April to broaden its SALUS Trial. The expansion includes the addition of high-risk patients and randomization against a commercial device, the Medtronic CoreValve.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now