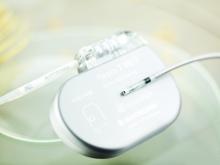
Scott & White Memorial — Temple for the first time implanted a new miniaturized, wireless monitoring sensor to help manage heart failure (HF). Scott & White Memorial is one of six hospitals in Texas and the first hospital in the Baylor Scott & White Health system to offer the device.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now