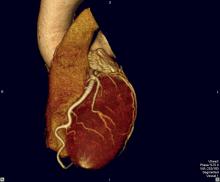
AstraZeneca announced full results from the PEGASUS-TIMI 54 study, a large-scale outcomes trial investigating Brilinta (ticagrelor) tablets plus low dose aspirin, at the American College of Cardiology (ACC) 64 th annual scientific session and expo in March. The study compared Brilinta to placebo plus low dose aspirin, for the chronic secondary prevention of atherothrombotic events in patients who had experienced a heart attack one to three years prior to study enrolment. Study results were simultaneously published in the New England Journal of Medicine online.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now