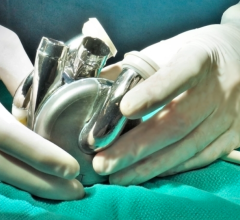
August 29, 2017 — Abbott announced it has received U.S. Food and Drug Administration (FDA) approval for its Full MagLev HeartMate 3 Left Ventricular Assist System. The HeartMate 3 system provides a new option for physicians managing advanced heart failure patients in need of short-term hemodynamic support (bridge-to-transplant or bridge to myocardial recovery). The system also provides patients living with their device new benefits that embody the evolution of left ventricular assist device (LVAD) therapy, such as improved blood flow in a pump that uses full magnetic levitation to reduce trauma to blood passing through the system.
More than 5.7 million people in the U.S. suffer from heart failure and approximately 915,000 new patients are diagnosed with the disease each year. For advanced heart failure patients who can no longer rely on earlier-stage treatment options, an LVAD can help their weakened heart pump blood through the body and provide crucial support as patients await further treatment, including heart transplants.
For patients who are not candidates for heart transplants or who will live with their device long-term, Abbott continues to offer HeartMate II, which is indicated for long-term or "destination therapy."
In developing the HeartMate 3 system, Abbott reduced the system's size while reimagining how blood passes through an LVAD. The system deploys Full MagLev technology to reduce trauma to the blood passing through the pump while optimizing blood flow. Improved blood flow can help minimize complications — such as pump thrombosis — that can be associated with LVAD therapy, ultimately improving the patient's quality of life.
U.S. approval of the HeartMate 3 system was supported by the MOMENTUM 3 clinical study. In that study, patients who received a HeartMate 3 system had a significant improvement in their heart failure status, an 83 percent increase in their walk distance and a 68 percent improvement in quality of life at six months. Patients receiving HeartMate 3 also had an 86 percent survival rate with freedom from disabling stroke and reoperation to replace the pump at six months.
"In the MOMENTUM 3 study, the HeartMate 3 system had no instances of suspected or established blood clotting within the pump at six months, which is a major milestone for those of us working tirelessly to improve clinical outcomes for patients living with advanced heart failure," said Mandeep R. Mehra, M.D., medical director of Brigham and Women's Hospital Heart and Vascular Center in Boston.
The MOMENTUM 3 study includes more than 1,000 patients with New York Heart Association (NYHA) Class IIIB or IV heart failure. Patients were followed for a short-term endpoint of six months and continue to be followed for a long-term endpoint of two years.
The HeartMate 3 system can pump up to 10 liters of blood per minute and is the only commercially-approved continuous flow implantable left ventricular assist system to utilize Full MagLev (fully magnetically-levitated) Flow technology, which allows the device's rotor to be "suspended" by magnetic forces, rather than bearings, with the goal of being able to more gently pass the blood cells through the pump. The magnets keep the rotor in place by calibrating tens of thousands of times per second to ensure it stays suspended and centered within the pump, no matter the speed settings used by a physician. This ensures the pump is performing effectively while continuing to deliver the best patient therapy possible.
The HeartMate 3 system also uses the industry's widest pump pathway, according to Abbott, designed so the blood cells are not damaged when passing through. The system also relies on a built-in "pulse" programmed to help ensure the blood continues to move through without becoming static, therefore reducing the risk of blood clot formation.
The HeartMate 3 system includes the LVAD pump as well as an external, wearable controller, driveline and battery system that powers the pump.
The system was CE Mark approved in Europe for both short-term and long-term support in October 2015.
For more information: www.abbott.com

 May 30, 2025
May 30, 2025