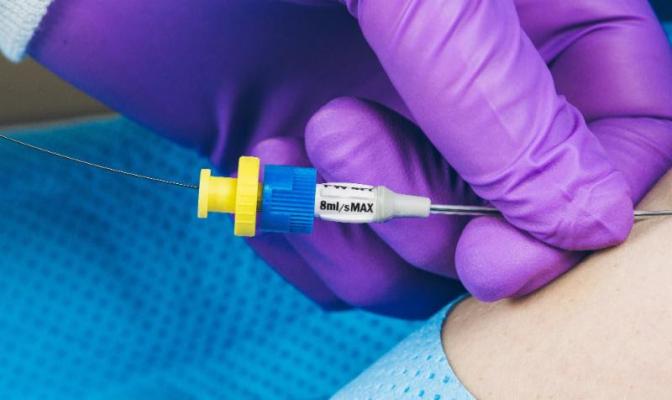
August 10, 2016 — Access Scientific introduced the PowerWand XL, a companion to the PowerWand All-in-One, in July. The Powerwand XL is designed specifically for clinicians who prefer to use a component-based, over-wire insertion technique similar to the Modified Seldinger Technique (MST) they currently use.
The XL technique feels similar to the MST, but XL advantages include:
- Improves clinical outcomes through atraumatic catheter insertion;
- Designed to reduce risk of deep-vein thrombosis by up to 500 percent. No trimming of catheter necessary;
- Reduces risk of back leaking. No peel-away sheath;
- Guidewire extends fully so the catheter does not drag across the intima of the vessel; and
- Faster and safer because of fewer steps.
As with MST insertions, the XL insertion technique utilizes a 21-gauge safety needle and guidewire to access the vessel. The XL accelerates line placement and makes it safer compared to MST, according to Access Scientific, by eliminating the need for a peelable introducer sheath. Instead, the proprietary PowerWand XL dilator and catheter are inserted directly over the wire and into the bloodstream. This technique eliminates the sheath-related steps in MST that can be associated with such risks as bleeding, back-leaking and vessel damage.
The All-in-One is inserted with the Accelerated Seldinger Technique (AST), which also improves on the MST in speed, number of steps and safety.
Other attributes of the PowerWand XL include:
- Size: 4Fr and 5Fr;
- Length: 8 and 10cm;
- Infection Prevention: Zero BSIs over 12,000 catheter-days;
- High Flow: Capable of delivering large volumes rapidly, 130-180 ml/min;
- Power Injectable: 325psi / 8ml per second;
- Blood Drawable: Allows for blood draws in most patients, 60 – 99 percent; and
- Patient Satisfaction: Provides patients with one-stick hospitalization.
For more information: www.accessscientific.com


 November 14, 2025
November 14, 2025 









