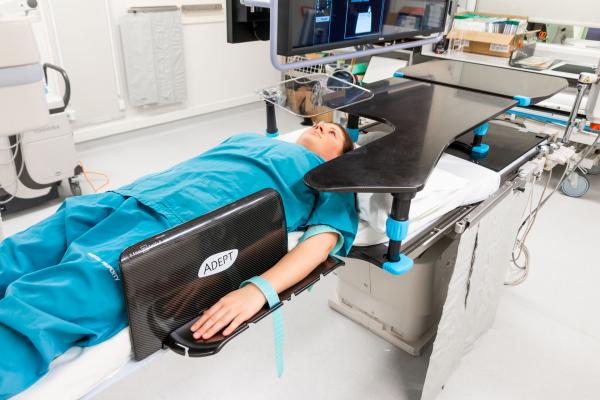
September 3, 2019 — New Zealand-based Adept Medical announced the launch of the Antegrade IR Platform. Clinically driven, it is placed to provide an ideal work surface for antegrade femoral approach during interventional radiology vascular procedures. The device sits within the company’s existing range of access and patient positioning devices designed for interventional radiology, cardiology and vascular procedures.
Designed to provide a stable, radiolucent, height- and length-appropriate work surface for supporting lightweight equipment during the antegrade femoral approach, the Antegrade IR Platform offers clinical benefits to the current practice of laying procedural equipment on the mattress and patient.
The narrow portion of the Antegrade IR Platform extends alongside the patient towards the femoral artery, offering a steady platform for the clinician to rest their wrists on during wire manipulation. It can be set up at two different lengths and various heights according to the patient and procedure, providing flexibility.
Ideally used in conjunction with the Adept Medical Drape Support (AM1000) and ArmSure (AM0600), these two products offer drape management, patient comfort and security during interventional radiology procedures.
Crafted from carbon fiber composite and high-performance engineering plastics, the Antegrade IR Platform is lightweight yet strong, and has a high resistance to chemical attack from commonly used cleaning products.
For more information: www.adeptmedical.co.nz


 November 14, 2025
November 14, 2025 









