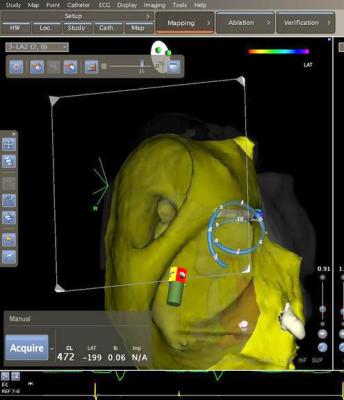
July 7, 2015 - Biosense Webster Inc. announced the launch of the Confidense Module, an innovative technology that streamlines the creation of real-time 3-D maps of a patient's cardiac structures during catheter ablation procedures. Utilizing an advanced, proprietary algorithm, the module enhances the process for validating information, or "points," acquired during multi-electrode mapping, enabling electrophysiologists to focus their time and expertise on the most relevant data during the mapping process.
"While high-density maps generated through multi-electrode mapping provide the most comprehensive and accurate image of the cardiac structures, this process can prove to be time-intensive," said Vias Markides, M.D., director of the Heart Division at Royal Brompton Hospital, London, United Kingdom. "The Confidense Module streamlines the mapping and validation process, which has the potential to reduce overall mapping, annotation and re-annotation time. I'm pleased that continued innovation in this space is enabling us to realize important clinical efficiencies while preserving the critical data and accuracy we have come to rely on from the Carto System."
Multi-electrode mapping allows for the rapid acquisition of a large quantity of data used to generate detailed, high-density maps on the Carto 3 System. Previously, validating the information acquired through multi-electrode mapping required a very detailed manual process, but the Confidense Module enhances the 3-D mapping experience with several novel features that simplify and accelerate map creation while maintaining accuracy.
The module also:
- Provides a real-time assessment of whether catheter electrodes are in proximity with heart tissue while selectively utilizing the points closest to the tissue to generate a high-quality 3-D map;
- Enhances the consistency of quality data acquisition with an advanced algorithm; and
- Accurately identifies and quickly evaluates points whose timing is judged to be inconsistent in comparison with neighboring points, which are then flagged and immediately brought to the physician's attention.
The Confidense Module is CE Marked in Europe and received U.S. Food and Drug Administration (FDA) 510(k) clearance in the United States.
For more information: www.biosensewebster.com


 February 06, 2026
February 06, 2026 









