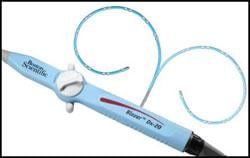
May 18, 2009 – Boston Scientific showcased its new Blazer Dx-20, a bidirectional duodecapolar diagnostic catheter, during Heart Rhythm 2009 last week in Boston.
The company said the catheter offers bidirectional steering for easier access and advancement in the coronary sinus. Backsteering helps secure the catheter’s position within the coronary sinus, a curve-lock helps stabilize the tip, and it is designed with torque response to help provide more predictable handling. The catheter has a soft, extended distal tip designed to lower the risk of vessel injury or perforation. The softer tip also reportedly conforms better to the anatomy, allowing better electrode contact.
The company said the Blazer Dx-20 leaves the isthmus area unobstructed and reduced the number of diagnostic catheters that are required.
The catheter comes in a 7 Fr. shaft size and in two curve styles – a super large curve measuring 56 mm and medium curve measuring 25 mm.
For more information: www. Bostonscientific.com/electrophysiology


 October 28, 2025
October 28, 2025 









