The first use of the OffRoad System was performed by J.A. Mustapha, M.D., director of Cardiac Catheterization Laboratories, director of Endovascular Interventions and director of Cardiovascular Research at Metro Health Hospital in Wyoming, Mich.
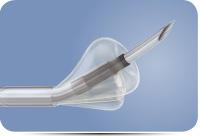
The OffRoad Re-Entry Catheter System is intended to help physicians navigate around complete arterial blockages by traveling within the subintimal space. Once the catheter has passed the blockage, a conical-shaped positioning
balloon is used to expand the subintimal space and direct a micro-catheter lancet to re-enter the vessel. This allows the physician to position a guidewire across the occlusion and to then treat the blockage using traditional endovascular techniques such as angioplasty and stenting.
Boston Scientific received U.S. Food and Drug Administration (FDA) clearance in late 2013, following favorable results from the Re-ROUTE
clinical trial. In the trial, investigators used the OffRoad System to successfully navigate around challenging CTOs in 84.8 percent of the enrolled patients, exceeding pre-specified trial goals.
For more information: www.bostonscientific.com