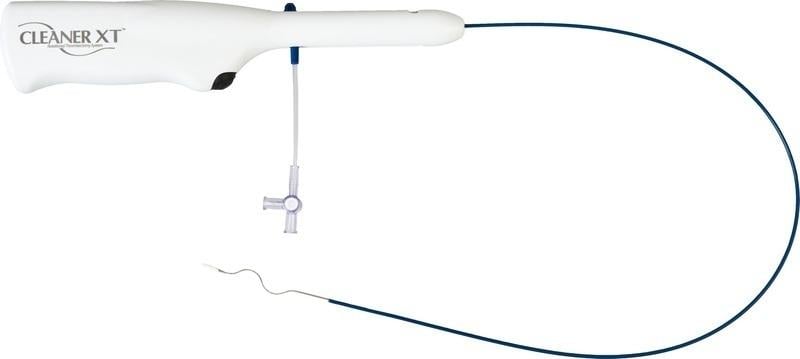
May 8, 2014 — Argon Medical Devices Inc. began marketing the CelanerXT rotational thrombectomy system as a new addition to the Cleaner family of dialysis products. With increased power and torque for cleaning wall-adherent thrombus, and the ability to be introduced through a 6-French sheath, CelanerXT combines torque with trackability. Its innovative sinusoidal wire design is radiopaque and conforms to varying lumen diameters, actively cleaning wall-adherent thrombus.
Argon introduced the CelanerXT rotational thrombectomy system at the 2014 Society of Interventional Radiology meeting in San Diego.
The CelanerXT rotational thrombectomy system was designed by Rex Medical LP for mechanical declotting of native vessel dialysis fistulae and synthetic dialysis access grafts. According to the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), almost 400,000 patients undergo dialysis each year. With this product launch, patients who require thrombectomy will now have access to a more efficient and effective therapy option.
For more information: www.argonmedical.com


 November 21, 2022
November 21, 2022