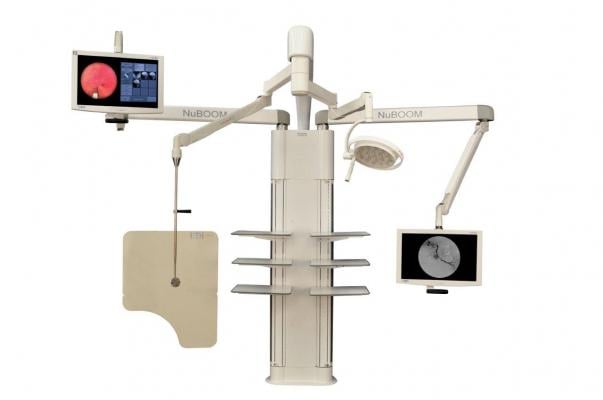
November 26, 2014 — CompView Medical (CVM) debuts the M2u and M4u, its latest design of NuBOOM, an all-in-one equipment manager, visualization and ergonomic boom system. The M2u and M4u are fully featured for the needs of cost-conscious interventional radiology (IR) applications. The U-Series NuBoom will be on display at the GE Healthcare booth (#4039) at the Radiological Society of North America (RSNA) 2014 meeting, Nov. 30–Dec. 4, 2014 in Chicago.
NuBOOM can be installed over a weekend and is light enough that it generally does not impact floor loading. It supports up to three booms for display suspension, radiation shields and surgical luminaires, eliminating ceiling construction. Its touchscreen control system enables access to fluoro, ultrasound, vitals, electronic medical records (EMRs), MIS cameras, picture archive and communication systems (PACS) and other inputs.
Traditionally, displays have been mounted into large matrix arrays. More recently, large 50-inch+ displays have increasingly been used inside IR ORs, precluding ergonomic positioning for comfortable viewing by the interventionist. NuBOOM solves that problem by mounting the screens from long adjustable booms/arms. An LED procedure light and a transparent radiation shield can also be suspended from the NuBOOM.
The NuBOOM is designed to complement the mobile C-Arm for IR. They are lighter-weight than most fixed equipment and don’t require extensive construction during installation. The inherent flexibility of a modular approach also enables the operating room to be utilized for other surgical specialties, should IR not be able to fully book all the available block time.
For more information: www.nuboom.com


 October 24, 2025
October 24, 2025 









