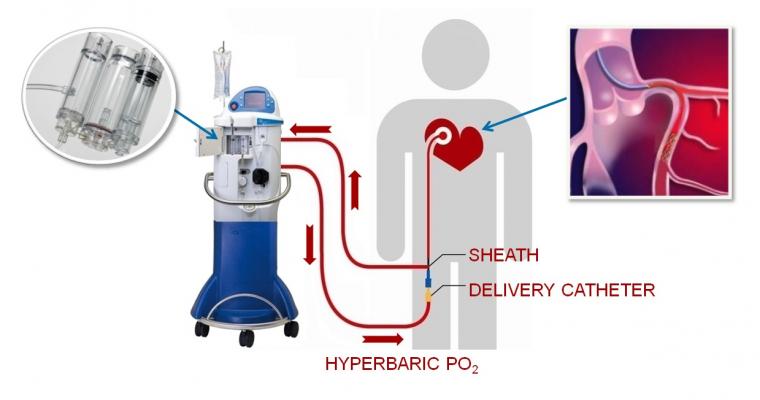
September 29, 2017 — TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted the premarket approval (PMA) application for its Supersaturated Oxygen (SSO2) Therapy system. The second-generation SSO2 Therapy system is designed to reduce infarct size and thereby improve outcomes in anterior acute myocardial infarction (AMI) patients treated within six hours of symptom onset.
Although percutaneous coronary intervention (PCI) is the standard of care in treating AMI, for many patients it does not sufficiently reduce infarct size to achieve maximum clinical benefit. In SSO2 Therapy, the patient’s blood is supersaturated with oxygen and then returned directly to the targeted ischemic area of the heart through a small catheter. Adjunctive to PCI, SSO2 Therapy is intended to salvage heart muscle and reduce infarct size.
The PMA application includes data from the IC-HOT (Evaluation of Intracoronary Hyperoxemic Oxygen Therapy) study, a confirmatory study that enrolled 100 patients at 15 investigational centers in the United States. The primary objective of the IC-HOT study was to collect confirmatory data supporting the safety and effectiveness of SSO2 Therapy in the treatment of anterior ST-elevation AMI patients who have undergone successful PCI with stenting within six hours of experiencing AMI symptoms.
For more information: www.therox.com


 November 14, 2025
November 14, 2025 









