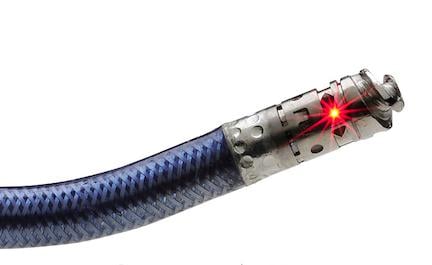
November 16, 2012 – The U.S. Food and Drug Administration (FDA) has granted market clearance for the Avinger Inc. Ocelot optical coherenace tomography (OCT) catheter to help cross chronic total occlusions (CTOs) in patients with peripheral artery disease (PAD).
The Ocelot catheter, supported by the Lightbox console, allows physicians to see from inside an artery during the actual procedure using forward-looking OCT. In the past, operators have had to rely solely on X-ray angiography and touch to guide catheters through complicated blockages. The current method makes for imprecise vessel navigation, leading to long procedure times and possible complications, such as vessel perforation. With Ocelot, physicians can more accurately navigate through CTOs thanks to the addition of imaging what is ahead of the catheter.
Avinger successfully completed enrollment in its CONNECT II global clinical trial in June 2012. The Ocelot System demonstrated a CTO crossing success of 97 percent with a 98 percent freedom from major adverse events (MAEs).
“It has taken my entire career to get to this moment,” said John B. Simpson Ph.D., M.D., Avinger founder and CEO. “Incorporating intravascular OCT into therapeutic devices has been the biggest priority here at Avinger. We have amazing investors who have allowed us to demonstrate how revolutionary Avinger can be. I’m so proud of our employees, all the physicians and hospitals around the world that helped us bring this amazing technology to the patients in the U.S.”
PAD is an unrecognized epidemic that affects between eight and 12 million adults in the United States and 30 million people globally. It is caused by a build-up of plaque in the arteries that blocks blood flow to the legs and feet. “Often times, bypass surgeries or amputations are the recommended solutions. Ocelot can help save patients from such dire circumstances and patients can be back on their feet in days,” continued Dr. Simpson.
A total of 122 patients from both Europe and the U.S. took part in the Ocelot global clinical trial, the results of which led to today’s FDA clearance.
Often dismissed as normal signs of aging, symptoms of PAD include painful cramping, numbness, or discoloration in the legs or feet. Hospitalization costs of PAD alone are estimated to exceed $21 billion annually, largely due to late detection and patients experiencing a decreased quality of life from invasive bypass surgery or amputation.
Because some blockages can become so severe and difficult to penetrate with traditional catheters, patients often are subjected to extremely invasive bypass surgeries that result in even higher health risks and lengthy, painful recoveries.
For more information: www.avinger.com


 November 14, 2025
November 14, 2025 









