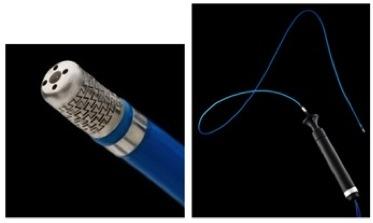
January 23, 2014 — The U.S. Food and Drug Administration (FDA) gave market clearance to Irvine Biomedical Inc., a St. Jude Medical company, for its Therapy Cool Flex Ablation Catheter and IBI 1500T9-CP v.1.7 Cardiac Ablation Generator.
The Therapy Cool Flex is a steerable, deflectable, irrigated
catheter used to treat atrial flutter by finding the rhythm disturbance source and
ablating small areas of the heart tissue. The catheter takes energy from an external source, the IBI1500T9-CP V1.7 Cardiac Ablation Generator, to a point in the right side of the heart.
The pattern of the ablation catheter laser-cut electrode tip allows flexibility and provides a more uniform distribution of saline fluid to the ablation site.
Cardiac catheter ablation can cure typical atrial flutter and restore a normal heart rhythm. In other cases, it can reduce the frequency of episodes a patient experiences. In a
clinical study involving 179 patients, the abnormal rhythm was corrected in 177 patients (98.8 percent) at least 30 minutes after treatment, and it remained corrected after 3 months in 150 patients.
The device is contra-indicated in patients who have an active systemic infection, a blood clot attached to the inside of the heart or an incision in the atrium or ventricle from within the last four weeks.
For more information: www.accessdata.fda.gov