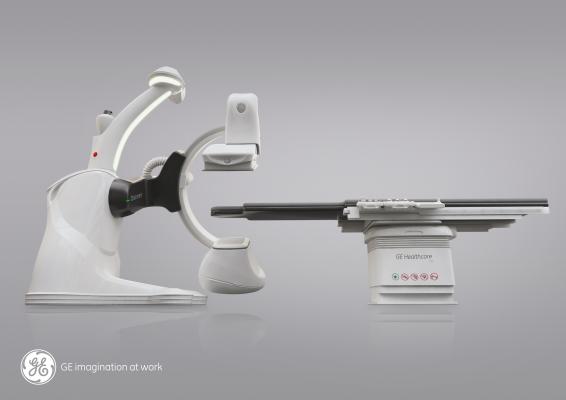
FDA Clears GEs Discovery IGS 740 Mobile Angiography System
May 7, 2014 — GE Healthcare received U.S. Food and Drug Administration (FDA) clearance for its Discovery IGS 740, a new rail-free mobile angiography system with a 41x41 cm detector. The Discovery IGS 740 is the first of this kind of mobile angiography system in the industry to receive FDA approval.
This new imaging system puts interventional radiologists at the center of their procedures. The rail-free design allows healthcare professionals ample access to the patient while freeing clinical teams from the constraints of fixed ceiling-mounted system rails. Its wide bore C-arm and dedicated arm-imaging positions create ease in imaging the anatomy of interest and full patient access from the left or right. A rotating laser continuously scans the room so the system knows where it is at all times.
By eliminating the ceiling rails, installation is simplified for flexibility in designing the room and positioning ceiling-mounted ancillaries (monitors, radshields, lights) where healthcare professionals need them.
The Discovery IGS 740 is equipped with two customizable parking positions to accommodate multiple room sizes and shapes. The 41x41-cm detector enables imaging of large organs, such as the liver and simultaneous coverage of both legs. The wide-bore C-arm helps interventional radiologists image large patients and conveniently perform off centered 3-D acquisitions. In addition, the system comes equipped with more than 20 advanced applications, such as FlightPlan for Liver, which helps clinicians identify tumor-feeding vessels in a few clicks, and be selective during liver embolizations.
For more information: newsroom.gehealthcare.com


 November 14, 2025
November 14, 2025 









