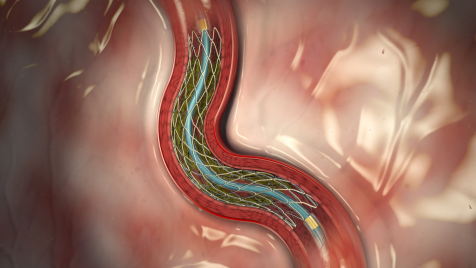
February 20, 2012 — Medtronic announced U.S. Food and Drug Administration (FDA) approval of the Resolute Integrity drug-eluting stent (DES) for the treatment of coronary artery disease (CAD).
The new heart device’s FDA approval stems from the results of a global series of studies involving the Resolute DES, which showed consistently powerful clinical performance across a broad spectrum of patients –– including those with diabetes, a common contributor to coronary artery disease that complicates treatment. The Resolute DES uses the same drug-and-polymer combination as the Resolute Integrity DES.
The Resolute Integrity DES builds on the success of the market-leading Integrity bare metal stent. The Integrity platform’s rapid adoption in the United States is the result of a proprietary engineering advance called continuous sinusoid technology (CST).
CST encompasses one continuous, single strand of wire that is molded into a sinusoidal wave and then wrapped in a helical pattern and laser-fused at certain points, making each stent comparable to a flexible spring.
“The Resolute Integrity DES offers several notable benefits, starting with outstanding deliverability, which means it’s exceptionally easy to navigate the stent on the delivery system through the coronary vasculature to the narrowed arterial segment that requires treatment,” explained Martin B. Leon, M.D., director of the center for interventional vascular therapy at New York-Presbyterian/Columbia University Medical Center, founder and chairman emeritus of the Cardiovascular Research Foundation, and a principal investigator (PI) of the RESOLUTE US clinical study. “Its approval by the FDA is based on the impressive performance of the Resolute DES in a wide variety of patients. With the device’s compelling combination of deliverability, efficacy and safety, not to mention that it is the first DES approved for patients with diabetes, the Resolute Integrity DES promises to gain rapid acceptance in cath labs nationwide.”
Clinical Performance
The global RESOLUTE clinical program consisted of a large randomized controlled trial and a series of confirmatory single-arm studies involving nearly 250 sites in 32 countries. In total, the program enrolled more than 5,100 patients who received a Resolute DES; about a third (1,535) of these patients had diabetes, a proportion that mirrors the United States patient mix.
RESOLUTE US enrolled 1,402 patients across 128 United States-based clinical trial sites. It was led by Leon and his co-principal investigators: Laura Mauri, M.D., chief scientific officer of the Harvard Clinical Research Institute and interventional cardiologist at Brigham and Women’s Hospital in Boston, Mass.; and Alan Yeung, M.D., director of interventional cardiology at Stanford University School of Medicine in Palo Alto, Calif.
At one year of follow-up in RESOLUTE US, the results included low rates of target lesion failure (TLF, 4.7 percent), clinically-driven target lesion revascularization (TLR, 2.8 percent) and definite/probable stent thrombosis (def/prob ST, 0.1 percent). These results were achieved despite 34 percent of the patients in the study having diabetes, which typically drives higher event rates.
One year of follow-up in a pre-specified analysis of patients with diabetes who received a Resolute DES as participants in the Resolute clinical program also demonstrated low rates of TLF (6.6 percent), TLR (3.4 percent) and def/prob ST (0.3 percent).
In two separate large randomized controlled trials, the Resolute DES matched the safety and effectiveness of Abbott Laboratories’ Xience V DES, which represents the market-leading DES platform in the United States.
The RESOLUTE All Comers study, sponsored by Medtronic, enrolled nearly 2,300 patients at 17 centers and was led by Prof. Patrick Serruys, M.D., Ph.D., director of the Thoraxcenter at Erasmus University in Rotterdam, the Netherlands; Prof. Stephan Windecker, M.D., with University Hospital in Bern, Switzerland; and Prof. Sigmund Silber, M.D., of the Heart Catheterization Centre in Munich, Germany. The one- and two-year results of RESOLUTE All Comers were published in The New England Journal of Medicine and The Lancet, respectively.
While not part of the FDA dataset, the TWENTE study, supported jointly by Medtronic and Abbott Laboratories, enrolled nearly 1,400 patients at a single center and was led by professor Clemens von Birgelen, M.D., Ph.D., co-director of the Department of Cardiology at Thoraxcentrum Twente and professor of cardiology at the University of Twente in the Netherlands. Prof. von Birgelen presented the one-year results of TWENTE at the 2011 Transcatheter Cardiovascular Therapeutics (TCT) meeting. The results are also reported in a recent issue of the Journal of the American College of Cardiology.
“The new Resolute Integrity DES comes to U.S. cath labs with compelling clinical evidence and a highly differentiated stent platform,” said Sean Salmon, president of Medtronic’s coronary and renal denervation business. “Our next-generation zotarolimus-eluting coronary stent has gained wide global acceptance for its remarkable ability to successfully meet clinical and anatomic challenges that interventional cardiologists confront in their everyday practice. We are excited to provide this important advanced technology to U.S. patients and practitioners.”
With the approval of the Resolute Integrity DES, United States patients with both CAD and diabetes now have access for the first time to a medical device that has been approved by the FDA as a treatment option specifically studied and clinically validated for their particularly complex and potentially life-threatening health conditions. Historically, patients with diabetes who undergo percutaneous coronary intervention (PCI) have been a difficult-to-treat patient population. They tend to have smaller and often tortuous arteries, longer lesions, diffuse disease and a higher rate of treatment failures including relatively high rates of repeat procedures and stent thrombosis.
U.S. Launch
The United States release of the Resolute Integrity DES also marks the first major product launch to leverage Medtronic’s entire United States Cardiac and Vascular Group (CVG) sales force, which includes nearly 3,000 field representatives whose collective objective is to serve the needs of hospital systems, integrated delivery networks and individual hospital administrators.
“No other medical device company has a United States field footprint as robust as Medtronic,” said Mike Coyle, president of Medtronic’s CVG. “We intend to use the launch of the Resolute Integrity DES to demonstrate the impact that our unrivaled scale can deliver for physicians, hospitals and patients looking for safe, effective and cost-effective solutions for cardiac and vascular diseases.”
In collaboration with leading clinicians, researchers and scientists, Medtronic offers the broadest range of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers worldwide.
Fore more information: www.medtronic.com


 November 14, 2025
November 14, 2025 









