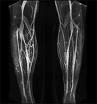
December 31, 2008 - The FDA today approved Vasovist Injection (gadofosveset trisodium), manufactured by EPIX Pharmaceuticals, is reportedly the first contrast imaging agent for use in patients undergoing magnetic resonance angiography (MRA).
Although MRA can be performed without the use of a contrast imaging agent, Vasovist is designed to provide a clearer image of blood vessels, compared to MRA without contrast, in patients who are suspected of having blockages or other problems with the blood vessels in their abdomen or limbs.
Vasovist is injected into a peripheral vein and no artery is punctured, thus the potential risks are fewer than injecting X-ray dye an artery, a procedure that may result in injury to vessel walls, blood clots, allergic reactions and potential kidney damage.
The active substance in Vasovist is gadolinium. The safety and efficacy of Vasovist was established in two clinical studies of patients with known or suspected aortoiliac disease. In the studies, patients underwent MRA with and without Vasovist and their scans were compared to standard X-ray pictures using contrast. MRA with Vasovist detected more arterial disease than MRA performed without Vasovist and the pictures were of improved technical quality.
The primary safety risks for Vasovist are allergic reactions and an ephrogenic systemic fibrosis, a rare syndrome that involves the thickening of the skin, joints, eyes and internal organs. The FDA issued a warning about this syndrome in May 2007 and information about it is included in a boxed warning for all drugs containing gadolinium, including Vasovist.
For more information: www.epixpharma.com


 August 17, 2023
August 17, 2023 








