

Images showing the platelet resistance of the CeloNova Cobra Pzf stent compared to Xience and Synergy.
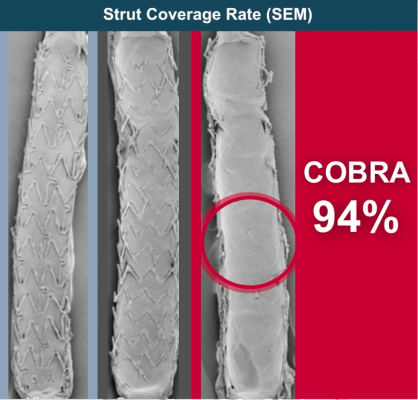
A comparison of endothelial stent strut coverage showing, in order left to right, Xience, Synergy, Cobra Pzf.

Highlights of the CeloNova Cobra Pzf stent.
March 2, 2017 — The U.S. Food and Drug Administration (FDA) cleared CeloNova BioSciences Inc. first-in-class Cobra PzF NanoCoated Coronary Stent System. Coated with a proprietary nano-thin polymer that is designed to be highly biocompatible, the Cobra PzF stent requires a minimum 30-day dual antiplatelet therapy (DAPT) regimen following intervention.[1]
Regulatory approval of the novel stent was based on findings from the pivotal PzF SHIELD clinical trial, which successfully met its primary safety and effectiveness endpoints at nine-month follow-up, demonstrating no stent thrombosis and low clinically driven target lesion revascularization (TLR) of 4.6 percent.[2]
Read the article “FDA OKs Second Randomized Trial for COBRA PzF Coronary Stent System.”
The Cobra PzF stent is indicated for improving coronary luminal diameter in patients, including those with diabetes mellitus, with symptomatic ischemic heart disease due to de novo lesions in native coronary arteries with reference vessel diameter (RVD) of 2.5-4 mm and lesion length of ≤24 mm.
“There continues to be an unmet clinical need for patients who may not be candidates for drug-eluting stents or longer term dual antiplatelet therapy,” said Donald Cutlip, M.D., principal investigator and professor of medicine at Beth Israel Deaconess Medical Center and Harvard Medical School in Boston. “Given the observed low rates of stent thrombosis and target lesion revascularization that need to be confirmed in future studies, the COBRA PzF stent system may hold potential unique benefit for these patients.”
The Cobra PzF combines a unique, highly deliverable cobalt chromium platform design with a proprietary Polyzene-F nano-thin polymer. When tested in pre-clinical studies, the ultra-pure, nano-thin characteristics of Polyzene-F nanocoating have shown thrombo-resistant, anti-inflammatory and rapid healing effects.[3,4,5,6]
“The stent’s Polyzene-F nanocoating is truly cutting-edge with good biocompatibility,” said Renu Virmani, M.D., president of the CV Path Institute in Gaithersburg, Md., the leading nonprofit medical research and education organization offering histology services studying cardiac and vascular diseases. “We continue to observe its thrombo-resistant and rapid endothelialization properties, which give us confidence to believe that COBRA PzF is a good stent option for patients who are at a high-risk for bleeding following coronary intervention.”[6]
“The FDA approval of the Cobra PzF Stent System marks a significant milestone for our company, as we bring a new category of stent with proven clinical promise to the U.S. market,” said Dennert Ware, Executive Chairman and acting CEO, CeloNova Biosciences. “We look forward to working with physicians throughout the country to integrate Cobra PzF into their care plans for the growing number of patients who would benefit from very low stent thrombosis, low TLR and a minimum 30 day DAPT regimen.”
Currently, the company is further studying the stent in the COBRA REDUCE trial, which began enrollment in February 2016. This randomized controlled trial will evaluate whether the Cobra PzF stent, with its novel Polyzene-F nanocoating and advanced thin-strut design, can help reduce bleeding as compared to drug eluting stents, by shortening the duration of DAPT to 14 days in patients who are at high-risk for bleeding and require treatment for coronary artery disease.
The Cobra PzF NanoCoated Coronary stent was awarded CE mark approval in 2012 and launched in Europe and the Middle East in 2013.
Read the article “CeloNova Enrolls First Patient in COBRA REDUCE Trial."
For more information: www.celonova.com
References:
1. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease. Accessed on February 16, 2017 at http://dx.doi.org/10.1016/j.jacc.2016.03.513.
2. Cutlip et al. Journal of the American College of Cardiology (JACC): Cardiovascular Interventions. 2017; V10; I2: 160-167.
3. Mrowietz C, Franke R, Seyfert U, et al. "Haemocompatibility of polymer-coated stainless steel stents as compared to uncoated stents." Clinical Hemorheology and Microcirculation. 2005; 32:89–103.
4. Sakakura K, Cheng Q, Fumiyuki O, et al. "Thrombogenicity of Novel Polyphosphazene Surface-modified Coronary Stent Compared To Standard Bare Metal Stent in Swine Shunt Model." Journal of the American College of Cardiology. 2013; 62(18): B244-B245. doi:10.1016/j.jacc.2013.08.1559 (28 day ex vivo swine arterio-venous shunt model)
5. Tamburino et al. Interventional Cardiology. 2011; 3: 451-460.
6. Data on File. Some data correlation between bench testing, animal studies and humans have not been determined.


 July 31, 2024
July 31, 2024 









