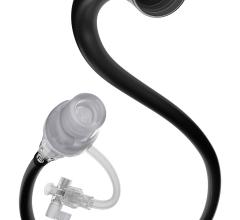
Concentric Medical has obtained FDA 510(k) clearance for its Merci L6 Retriever, a device that restores blood flow to ischemic stroke patients. The Merci Retriever is designed to restore blood flow by engaging, capturing and removing blood clots. It is a shaped wire constructed of nitinol, a memory wire, which allows delivery of the Retriever in linear form using standard catheterization techniques. A small puncture in the groin is used to introduce the Merci Retriever into an artery leading to the brain. Upon reaching the targeted area, the Retriever returns to its original shape when deployed. The Merci L6 Retriever joins the L family of retrievers, incorporating a 2.7-mm cylindrical helix with attached filaments. The filaments provide an additional mechanism for securing the clot during retrieval.
If you enjoy this content, please share it with a colleague
FDA Clears Neurovascular Retriever for Use in Stroke Patients
Related Content
Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston ...
Dec. 10, 2025 — Medtronic plc has announced the first commercial use of the Liberant thrombectomy system (Liberant) ...
Dec. 16, 2025 — A new study, published in the American Journal of Cardiology, found that the use of Computer Assisted ...
Nov. 3, 2025 — Penumbra, Inc. has announced additional results of the STORM-PE randomized controlled trial (RCT), which ...
Oct. 27, 2025 – Penumbra, Inc. has announced the results of the STORM-PE randomized controlled trial (RCT), which found ...
Sept. 2, 2025 — Imperative Care, Inc. has announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its ...
June 16, 2025 – Penumbra, Inc. recently announced the completion of enrollment in the STORM-PE clinical trial. This ...
March 24, 2025 — Imperative Care, Inc. recently announced U.S. Food and Drug Administration (FDA) 510(k) clearance of ...
Penumbra recently launched its Element Vascular Access System, a laser-cut hypotube sheath designed for venous ...
Nov. 5, 2024 —Penumbra, Inc. recently announced new data that demonstrate patients with intermediate-risk pulmonary ...


 January 19, 2026
January 19, 2026