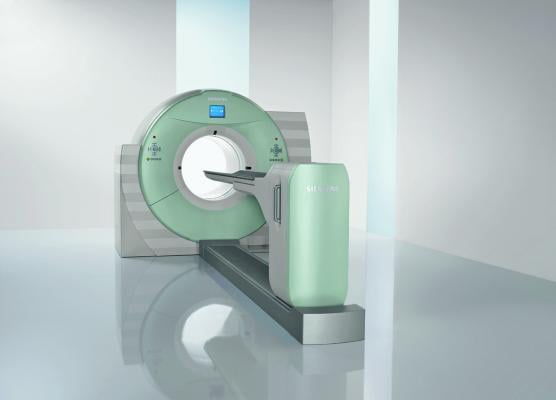
June 21, 2018 — Siemens Healthineers will announce U.S. Food and Drug Administration (FDA) clearance of four new system features for the Biograph mCT family of positron emission tomography/computed tomography (PET/CT) systems at the 2018 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI). These features, three of which are also available on the Biograph Vision PET/CT scanner, bring advanced clinical capabilities to healthcare providers, enabling them to expand precision medicine and transform the delivery of patient care.
The QualityGuard¹ feature self-calibrates the PET detector by tapping natural background radiation from the lutetium oxyorthosilicate (LSO) detectors to run daily and weekly quality control (QC) procedures during off-hours. This saves up to 30 minutes of staff time per day, according to Siemens. Self-calibration eliminates use of a radioactive source for daily and weekly manual calibration, thereby reducing the technologist’s radiation exposure in addition to eliminating the need to lift the radioactive source, which can weigh 25 to 30 lbs.
A second feature, FlowMotion Multiparametric PET Suite, is fully automated and delivers whole-body parametric PET images based on the Patlak reference tissue model. In a single examination and without the need for arterial blood sampling, FlowMotion Multiparametric PET Suite provides images of metabolic rate and distribution volume in addition to standard uptake value (SUV) images. FlowMotion Multiparametric PET Suite reduces the variability of quantitative measurements due to differences in uptake time, patient body size and blood glucose levels. This leads to better characterization of tracer uptake compared to SUV alone.
The OncoFreeze feature provides images that are virtually free of respiratory motion in the same time as a standard whole-body scan. Traditionally, respiratory gated images use only select information from a portion of the patient’s respiratory cycle; the remaining data from the PET/CT scan is discarded. OncoFreeze utilizes 100 percent of acquired information from the respiratory cycle and reduces acquisition time.
Finally, CardioFreeze corrects for respiratory and cardiac motion associated with cardiac PET/CT image acquisitions and can obtain the respiratory waveform just by analyzing PET data. Each cardiac gate is reconstructed with 100 percent of the data acquired. This eliminates variability from cardiac and respiratory motion, and improving visualization of myocardial tracer distribution, wall thickness and defect definition.
For more information: www.usa.healthcare.siemens.com
Read the related article "Nuclear Imaging Moves Toward Digital Detector Technology."
Reference
1. QualityGuard is currently under development on Biograph Vision and is not available for sale in the U.S. or any other country.


 January 05, 2023
January 05, 2023