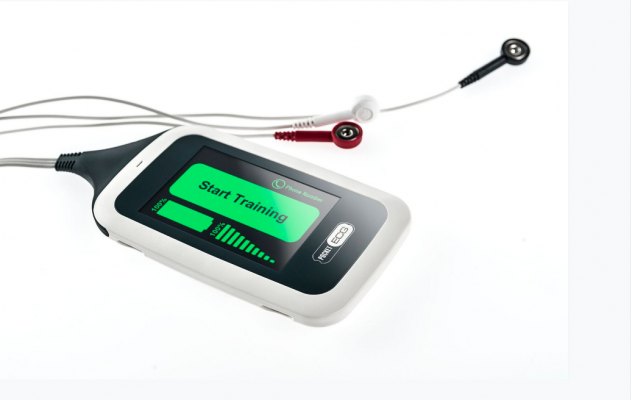
Now cleared by the FDA, PocketECG CRS is a new mobile cardiac rehabilitation system designed to provide high-quality ECG monitoring and automated arrhythmia detection during rehabilitation training. The device monitors a patient's heart rhythm and heart rate to safely guide the intensity and duration of rehabilitation exercises in real-time.
July 23, 2018 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the PocketECG Cardiac Rehabilitation System (CRS). The new mobile cardiac rehabilitation system is designed to provide high-quality ECG monitoring and automated arrhythmia detection during rehabilitation training. The clearance was announced by the maker MedicAlgorithmics and U.S. subsidiary Medi-Lynx Cardiac Monitoring LLC.
The device is currently approved and marketed in the European Union and patented in the U.S. (U.S. Patent No. 9,846,764) for use in low and high-risk cardiac patients.
"We are thrilled to now introduce PocketECG CRS to patients and clinicians in the U.S., providing a valuable monitoring tool with the potential to improve the effectiveness of cardiac rehabilitation training," said Marek Dziubinski, Ph.D., CEO of MedicAlgorithmics. "PocketECG CRS was built on our PocketECG arrhythmia monitoring solution platform, and includes new software and enhancements specifically designed for use during all phases of the cardiac rehabilitation process — from early mobilization during hospitalization to post-discharge and ongoing maintenance."
PocketECG CRS is a smartphone-sized device that monitors a patient's heart rhythm and heart rate during rehabilitation exercises to safely guide the intensity and duration of workouts in real-time. The system transmits the full disclosure ECG signal classifying every heartbeat to automatically detect abnormalities and arrhythmia. A built-in accelerometer allows clinicians to analyze the impact of physical activity on heart rate. All data are streamed via mobile telephony network to a secure online clinician portal and to a cardiac monitoring center for constant monitoring, evaluation, and urgent notifications.
Cardiac rehab or medically supervised exercise training is highly recommended by the American Heart Association (AHA) and shown to improve the health and recovery of patients with acute myocardial infarction or heart attack, chronic stable angina, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), cardiac valve surgery, stable, chronic heart failure and cardiac transplantation. The American Association of Cardiovascular and Pulmonary Rehabilitation estimates that at least 3.8 million patients are eligible to receive cardiac rehabilitation.
This device technology is part of a larger trend in remote ECG monitoring devices. Read more on these advances.
For more information: www.medicalgorithmics.com, www.medi-lynx.com


 November 12, 2025
November 12, 2025 









