March 12, 2015 — The U.S. Food and Drug Administration (FDA) approved the VenaSeal closure system to permanently treat varicose veins of the legs by sealing the affected superficial veins using an adhesive agent.
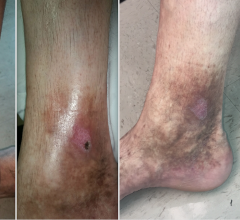
Veins contain one-way valves that open to let blood flow through and then shut to keep blood from flowing backward. When valves of the superficial system are weak or damaged, blood can back up and pool, which can cause varicose veins that are enlarged, swollen or twisted.
Varicose veins often cause no symptoms but some patients may experience mild to moderate pain, blood clots, skin ulcers or other problems, according to the National Heart, Lung and Blood Institute at the National Institutes of Health. If these issues occur, healthcare professionals may recommend treatment such as compression stockings or medical procedures to remove or close the affected veins.
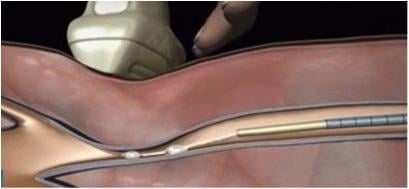
The VenaSeal system is intended for patients with superficial varicose veins of the legs that cause symptoms. The sterile kit is made up of an adhesive, a specially formulated n-butyl-2-cyanoacrylate, and delivery system components that include a catheter, guidewire, dispenser gun, dispenser tips and syringes.
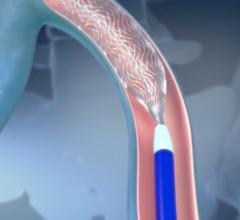
The device must be used as a system and differs from procedures that use drugs, laser, radio waves or cuts in the skin to close or remove veins. A trained healthcare professional inserts the catheter through the skin into the diseased vein to allow injection of the VenaSeal adhesive, a clear liquid that polymerizes into solid material. The healthcare professional monitors proper placement of the catheter using ultrasound imaging during delivery of the adhesive into the diseased vein to seal it.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive, thereby giving patients another treatment option for this common condition,” said William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health. “Because the VenaSeal system does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising.”
The FDA reviewed data for the VenaSeal system in a premarket approval application. Data supporting the FDA approval included results from three clinical studies sponsored by the manufacturer. The U.S. clinical study assessed the safety and effectiveness of the VenaSeal system in 108 participants compared to radio-frequency ablation in 114 participants. The trials showed the device to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs.
The VenaSeal system should not be used in patients who have a known hypersensitivity to the VenaSeal adhesive, acute inflammation of the veins due to blood clots, or acute whole-body infection. Adverse events observed in the trial — and generally associated with treatments of this condition — included vein inflammation (phlebitis) and burning or tingling (paresthesia) in the treatment zone.
The VenaSeal Closure system is manufactured by Covidien LLC, based in Morrisville, North Carolina.
For more information: www.fda.gov


 September 10, 2025
September 10, 2025