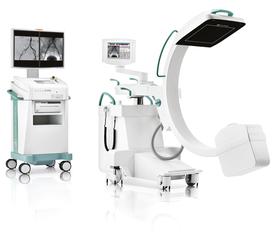
February 6, 2014 — Ziehm Imaging received U.S. Food and Drug Administration (FDA) clearance to market its new generation Ziehm Vision RFD
C-arm for pediatric and other interventional operating room procedures.
The Ziehm Vision RFD C-arm offers fully motorized movement on four axes to maximize image quality while minimizing procedural dose. It was cleared for a range of
image procedures including specific intended uses in pediatric imaging.
Ziehm Imaging supplyies 65 percent of the C-arm market in Europe.
For more information: www.ziehm.com