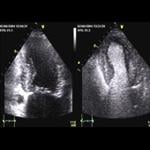
July 12, 2010 – GE Healthcare Medical Diagnostics today announced the market reintroduction of Optison (Perflutren Protein-Type A Microspheres Injectable Suspension, USP), a diagnostic ultrasound contrast agent for use in select echocardiograms. Optison is indicated for use in patients with suboptimal echocardiograms to opacify the left ventricle and to improve the delineation of the left ventricular endocardial borders. The safety and efficacy of Optison with exercise stress or pharmacologic stress testing have not been established.
Optison is an FDA-approved ultrasound contrast agent available in a ready-to-use formulation, providing the benefits of fast preparation time, ease of use, portability, and flexible dosing. Optison was approved by the U.S. Food and Drug Administration (FDA) in 1998. In June 2009, GE Healthcare was no longer able to supply Optison to the market due to manufacturing difficulties. Since that time, the company has conducted a thorough review of the manufacturing process and changes have been made to minimize any future disruptions to product supply.
The American Society of Echocardiography (ASE) recommends the use of contrast whenever two or more left ventricular wall segments are not seen on non-contrast images. In clinical studies supporting the marketing application of Optison, there was significantly increased left ventricular heart opacification and improved endocardial border delineation with Optison compared to non-enhanced echocardiograms.
“I am pleased that Optison is back on the market because it offers an important tool for improving the accuracy and reliability of echocardiograms, without unnecessarily exposing patients to ionizing radiation or dye,” said Steven B. Feinstein, M.D., FACC, FESC, professor of medicine and director of echocardiography, Rush University Medical Center, Chicago. “By improving the accuracy of echocardiograms, the use of ultrasound contrast agents also avoids redundant downstream testing."
Optison’s safety profile is supported by more than 1 million administered doses and nearly 12 years of clinical use, with only 16 serious adverse events reported since 1998. Of the reported adverse reactions following the use of Optison the most frequently reported were headache, nausea and/or vomiting, warm sensation or flushing, and dizziness.


 August 17, 2023
August 17, 2023 








