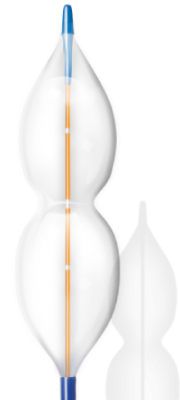
May 1, 2013 — InterValve Inc. announced that it has received 510(k) clearance to market the new V8 Aortic Valvuloplasty Balloon Catheter in the United States.
The V8 catheter is designed to be used in stand-alone balloon aortic valvuloplasty (BAV), and pre-dilation during transcatheter aortic valve replacement (TAVR) procedures.
“These procedures are limited today by the use of dated, conventional balloon technologies. The shape and material properties of the V8 balloon catheter are designed to enhance both procedures through reduction in balloon movement and optimized leaflet expansion without over distention of the annulus,” said Mark Ungs, chief executive officer of InterValve.
The balloon catheter features a figure-8 shape balloon that enables the bulbs at either end of the balloon to “lock” into either side of the aortic annulus. This design has the potential to reduce the risk of balloon movement during dilatation, thereby reducing procedure and ischemic time.
The non-compliant material maintains the figure-8 shape throughout inflation, which allows for leaflet hyperextension, to create maximum valve area opening, without increasing the risk of over-stretching the annulus.
Lastly, the catheter is designed to inflate and deflate rapidly to reduce the duration of blood flow obstruction across the valve.
For more information: www.intervalveinc.com


 June 13, 2024
June 13, 2024 









