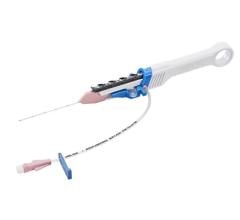
February 14, 2011 – A new bedside insertion kit and power-injectable catheter has been launched. The Morpheus Smart PICC insertion kit and 5 French Dual Lumen Peripherally Inserted Central Catheter (PICC), from AngioDynamics, are an extension of the company’s Morpheus Smart PICC line.
The 5 French power-injectable catheter is designed to provide a superior flow rate of 5 mL/second for purposes of CT imaging. This performance upgrade is coupled with the pushability needed for PICC placement with pliability that minimizes the risk of vessel trauma and phlebitis. It also employs Smart Taper technology, which is designed to reduce the risk of thrombosis by allowing the catheter’s diameter to quickly taper from 7 French to a precise 5 French.
“Our facility conducts more than 3,500 PICC placements per year,” said Julie Amicucci, chief, emergency center physician extenders and director of the PICC Team at William Beaumont Hospital, Royal Oak, Mich. “After switching to AngioDynamics, we’ve seen a reduced number of calls involving post procedure bleeding.”
Amicucci said her facility also will experience benefits from the new Morpheus Smart PICC bedside insertion kit, which includes upgraded premium safety components. Specifically, the safety components include a new sharps container, upgraded safety scalpel, safety needles, larger absorbent drapes to protect the sterile field and two ChloraPrep One-Step Applicators for maximum site preparation coverage. This new kit was designed to allow for safer, more efficient PICC placements at the patient’s bedside.
“When traveling from bedside to bedside in a clinical setting, it is the little things that make the difference,” she said. “The new AngioDynamics insertion kit is slimmer, which will allow us to store more kits per cart, and it has everything that is required for the procedure. This will allow us to provide better patient care by getting to patients faster, ultimately saving time and the hospital money.”
For more information: www.angiodynamics.com


 January 29, 2026
January 29, 2026