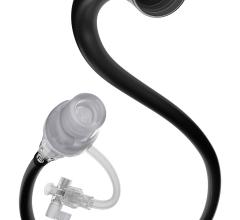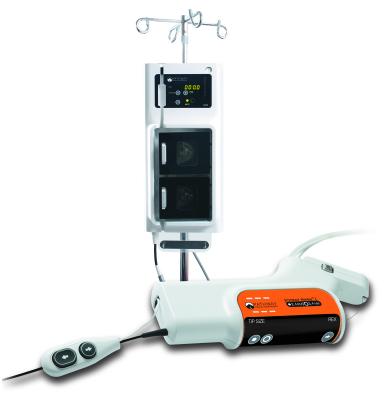

June 22, 2012 — Bayer HealthCare widens its portfolio of interventional solutions by introducing the Jetstream Navitus L atherectomy catheter. Positioned as the largest diameter rotational atherectomy device in the Jetstream catheter family, the Navitus L catheter can create a lumen 30 percent larger than the standard Navitus device.
Intended to treat above-the-knee peripheral artery disease (PAD), the Navitus L can be used in multiple lesions morphologies, including calcium and thrombus while providing differential cutting, to remove the lesion materials while preserving the soft vessel walls. Like all Jetstream atherectomy catheters, the Navitus L provides continuous, active aspiration and the unique front-facing cutting head. Like the original Navitus, the Navitus L offers two-stage cutting with expandable blade technology allowing physicians to treat both common femoral artery (CFA) and superficial femoral artery (SFA) disease with a single catheter.
For more information: www.interventional.bayer.com


 January 19, 2026
January 19, 2026