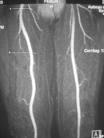
October 8, 2009 – Lantheus Medical Imaging Inc. said two oral presentations featuring gadofosveset trisodium, the first and only FDA cleared blood pool contrast agent for magnetic resonance angiography (MRA), were presented at MRA-Club 09: The 21st Annual International Conference on Magnetic Resonance Angiography. The company also announced today that ABLAVAR has been chosen as the brand name for gadofosveset trisodium. ABLAVAR (gadofosveset trisodium) is a blood pool contrast agent approved for magnetic resonance angiography to evaluate aortoiliac occlusive disease (AIOD) in patients with known or suspected peripheral vascular disease. The unique albumin-binding properties of ABLAVAR make it ideal for vascular imaging allowing multiple images to be obtained using a single, low dose injection. ABLAVAR enables the visualization of both arterial and venous blood vessels. ABLAVAR is clinically proven to produce high resolution MRA images, combining both dynamic (first pass) and steady state imaging, resulting in diagnostic accuracy comparable to X-ray angiography, the current standard of care for diagnosing vascular disease such as AIOD. ABLAVAR is indicated for use as a contrast agent in magnetic resonance angiography (MRA) to evaluate aortoiliac occlusive disease (AIOD) in adults with known or suspected peripheral vascular disease. The first presentation entitled, “An Update on the Clinical Experience with Gadofosveset,” was presented by Mark G. Hibberd, M.D., Ph.D., senior medical director, global medical affairs, Lantheus Medical Imaging Inc. This presentation included the review of safety and efficacy data from the clinical use of ABLAVAR in Europe and Canada (over three years of marketing data) and from the FDA approval of MS-325 in December 2008. The establishment of a new clinical registry in the United States was also announced. The presentation confirmed that ABLAVAR has not been associated with nephrogenic systemic fibrosis (NSF) to date. The second oral presentation, “A Re-analysis of MS-325 (Gadofosveset Trisodium) Clinical Trial Data in Support of U.S. FDA Approval,” was given by Edward Parsons, Ph.D., formerly of EPIX Pharmaceuticals Inc. and currently a consultant to Lantheus Medical Imaging Inc. This presentation, which reported a blinded, independent re-read of images from previous phase 3 studies, found that ABLAVAR demonstrated statistically greater sensitivity (detecting disease when present) compared with noncontrast MRA. The study also showed that ABLAVAR had noninferior specificity (excluding disease when not present) with noncontrast MRA1. Thus ABLAVAR MRA images provided diagnostic accuracy superior to non-contrast MRA and comparable to traditional X-ray angiography." “The data presented at the recent MRA Club meeting supports the safety and efficacy of ABLAVAR, a first-in-class imaging agent we are planning to introduce to the U.S. market in the coming months,” said Dr. Hibberd. “We are confident that ABLAVAR will make it possible for physicians to detect aortoiliac occlusive disease less invasively than with the current gold standard – X-ray angiography – and, importantly, without exposing the patient to ionizing radiation. Furthermore, ABLAVAR is used in a single low dose which allows both first pass and high resolution blood pool imaging.” In April 2009, Lantheus Medical Imaging acquired the U.S., Canadian, and Australian rights to gadofosveset trisodium (formerly known as MS-325) from EPIX Pharmaceuticals Inc. The product is approved in 38 countries worldwide and has been used in more than 60,000 patients in Europe. For more information: www.radiopharm.com


 August 17, 2023
August 17, 2023 








